ReadCube for Pharmacovigilance
Strengthen Drug Safety and Risk Monitoring with Comprehensive Literature Management and Alerts
Pharmacovigilance teams rely on a steady stream of clinical literature, safety reports, and regulatory data to monitor drug safety and manage risks. ReadCube’s literature management and monitoring platform provides a centralized hub for managing and tracking relevant publications, ensuring teams can quickly identify emerging risks and trends.
Key Features for
Pharmacovigilance
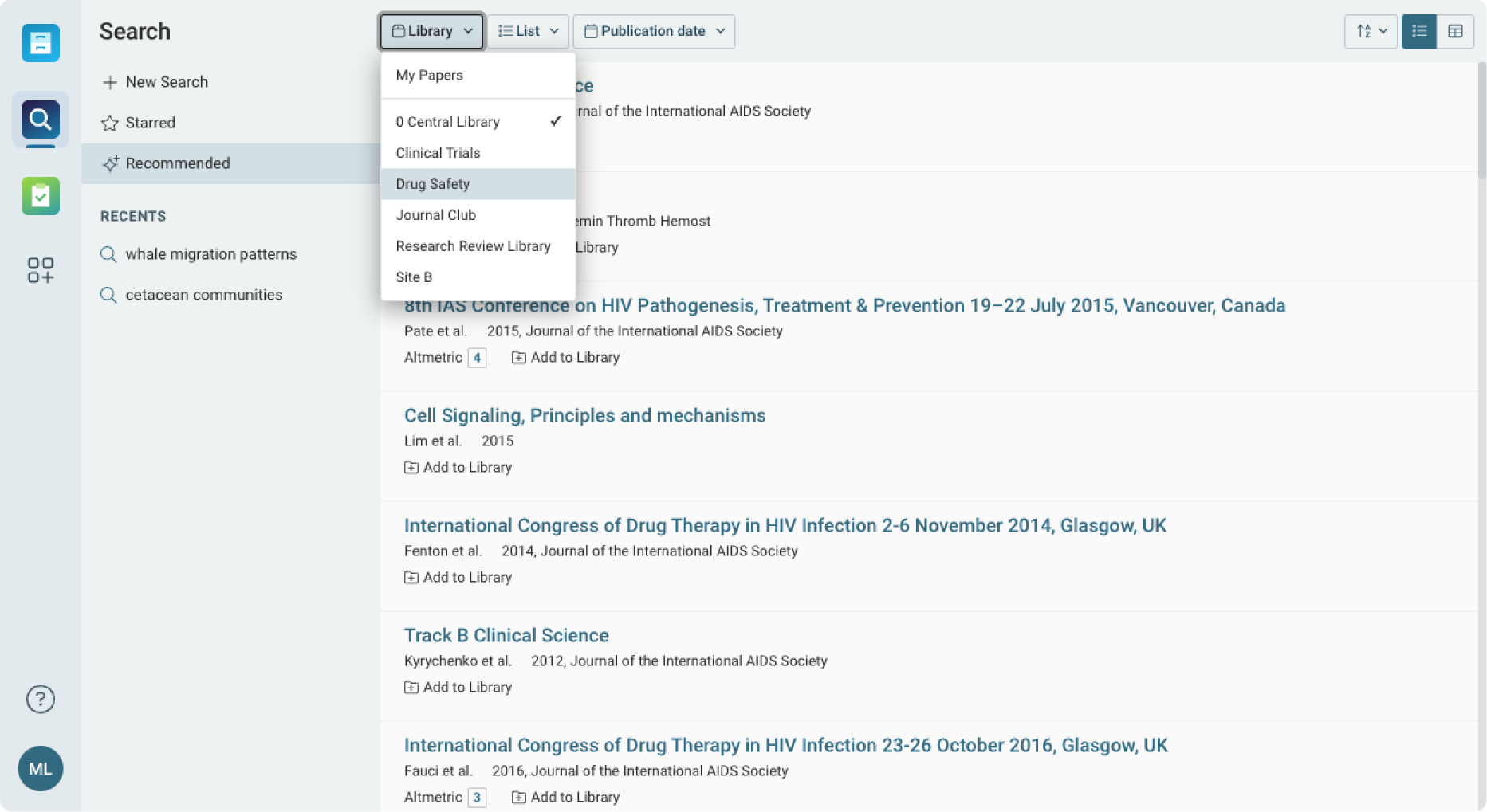
Organized Management of Drug Safety Literature
Centralize all drug safety data, including clinical reports, adverse event studies, and safety guidelines.
- Organize research by drug, therapy, or risk area for faster access to critical data.
- Share annotated reports and findings with regulatory teams or external stakeholders.
- Maintain consistent and up-to-date access to safety reports across global teams.
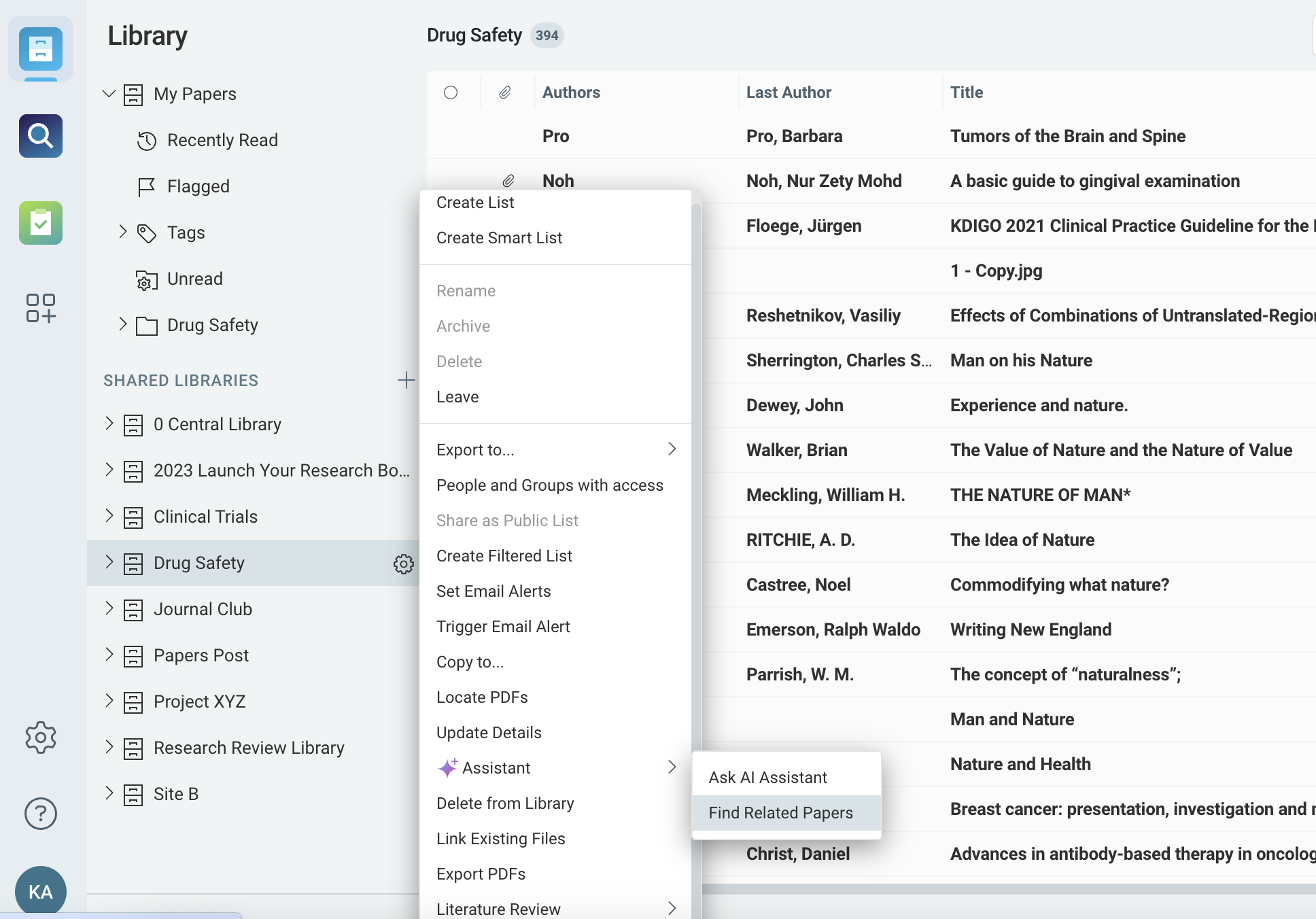
Real-Time Monitoring for Adverse Event and Risk Literature
Automatically monitor new studies, reports, or publications related to drug safety and adverse events.
- Set alerts for new findings related to drug efficacy, safety profiles, or regulatory updates.
- Stay informed about emerging risks in real time, allowing your team to react quickly to new safety concerns.
- Use AI-driven recommendations to uncover related safety literature that may inform risk management efforts.
Cross-Team Collaboration on Safety Insights
Improve collaboration between safety, clinical, and regulatory teams by sharing research libraries and key insights.
- Enable cross-functional teams to access shared safety reports and drug efficacy studies.
- Collaborate on monitoring drug safety trends and produce cohesive, data-backed reports for regulatory submissions.
Trying to stay on top of monitoring safety literature for multiple drugs while struggling to stay on top of new adverse event reports and regulatory updates?
Pharmacovigilance teams leverage ReadCube to automatically track new safety literature and adverse event reports across multiple drugs. They categorize literature by safety concern and geographic region to ensure regional teams receive the most relevant updates.
The AI-driven recommendations help them uncover additional related reports they might have missed. By sharing annotated safety reports and findings across regulatory and clinical teams, they ensure that risks are identified early and addressed before they escalate.