ReadCube for Medical Affairs
Optimize Medical Literature Management and Monitoring for Strategic Decision-Making
Medical Affairs teams are tasked with staying informed on the latest clinical research, regulatory updates, and scientific developments to make data-driven decisions. ReadCube offers a complete solution for managing, organizing, and monitoring medical literature, helping Medical Affairs professionals access critical information quickly and efficiently.
Key Features for
Medical Affairs
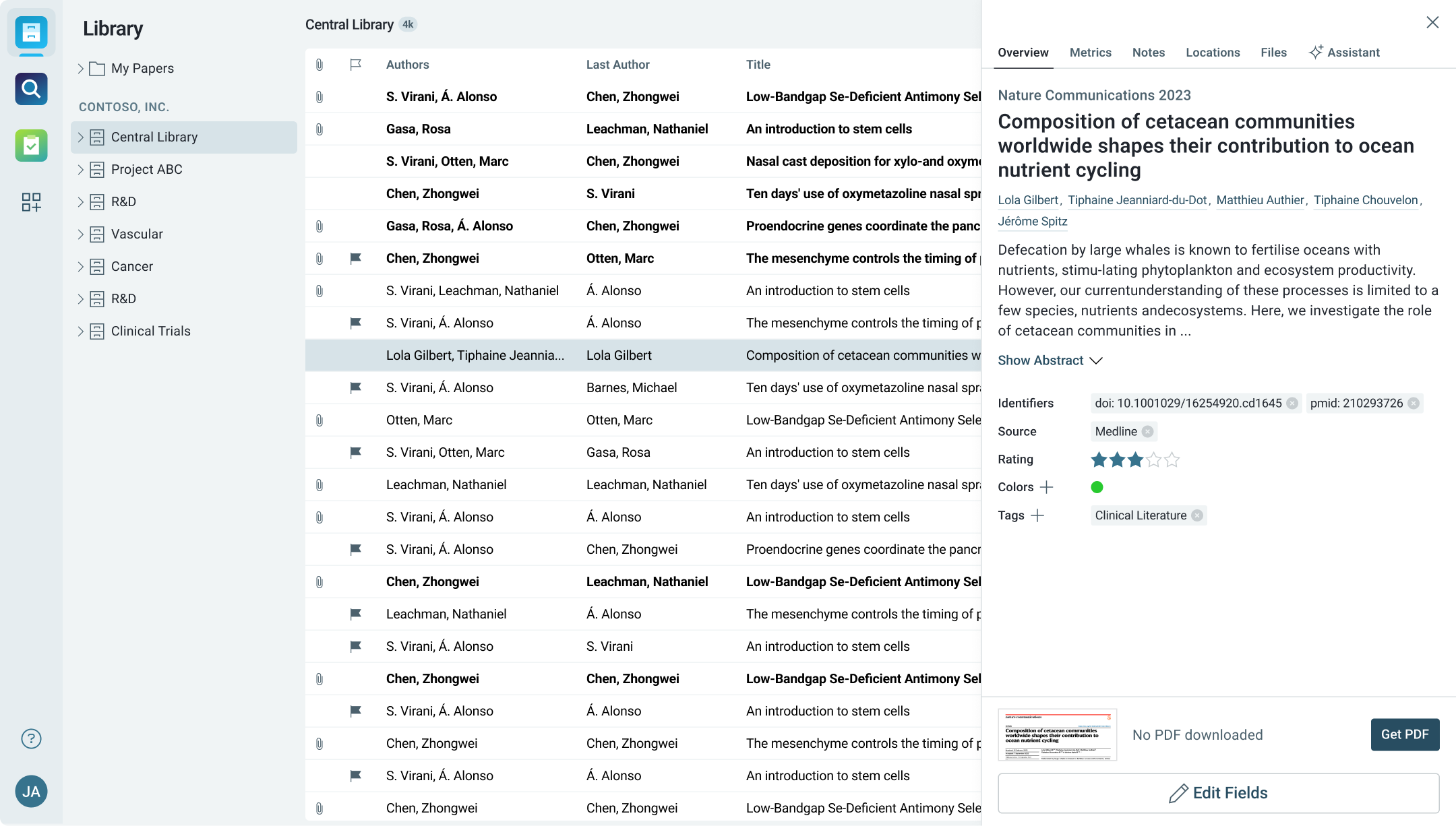
Centralized Literature Management for Clinical Data
Organize clinical studies, regulatory reports, and safety documentation in one easy-to-access library.
- Categorize research by therapeutic area, drug, or study phase for easy retrieval.
- Annotate and highlight key clinical findings for internal discussions or presentations.
- Ensure that all teams have access to up-to-date materials, streamlining compliance and approval processes.
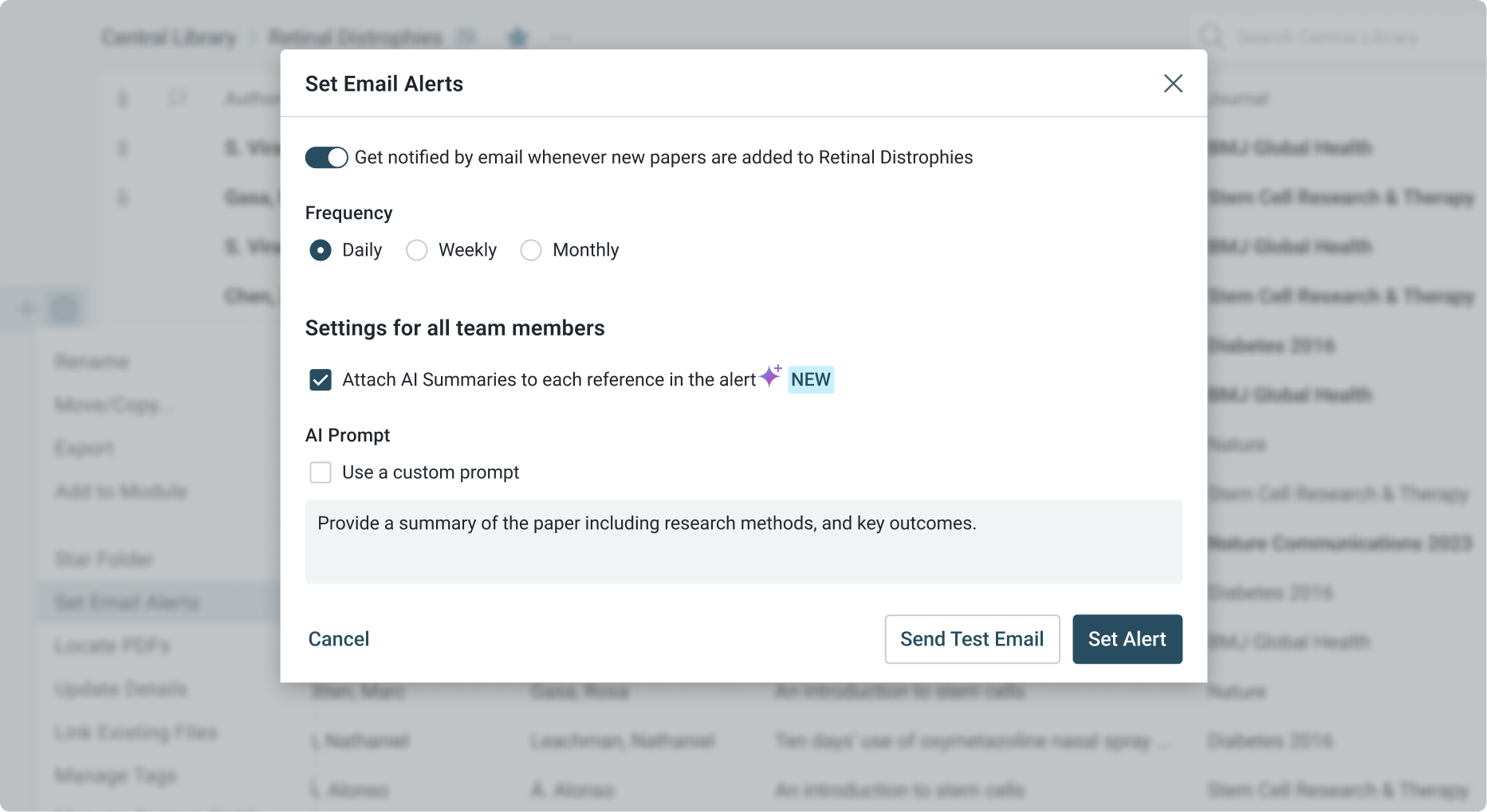
Continuous Monitoring of Regulatory Changes
Stay current with the latest medical literature and regulatory developments by setting up customized alerts.
- Automatically track new research in clinical trials, therapeutic areas, or drug safety.
- Monitor regulatory updates to ensure your team remains compliant with the latest standards and guidelines.
- Discover emerging trends and new treatment options that could inform strategy and decision-making.
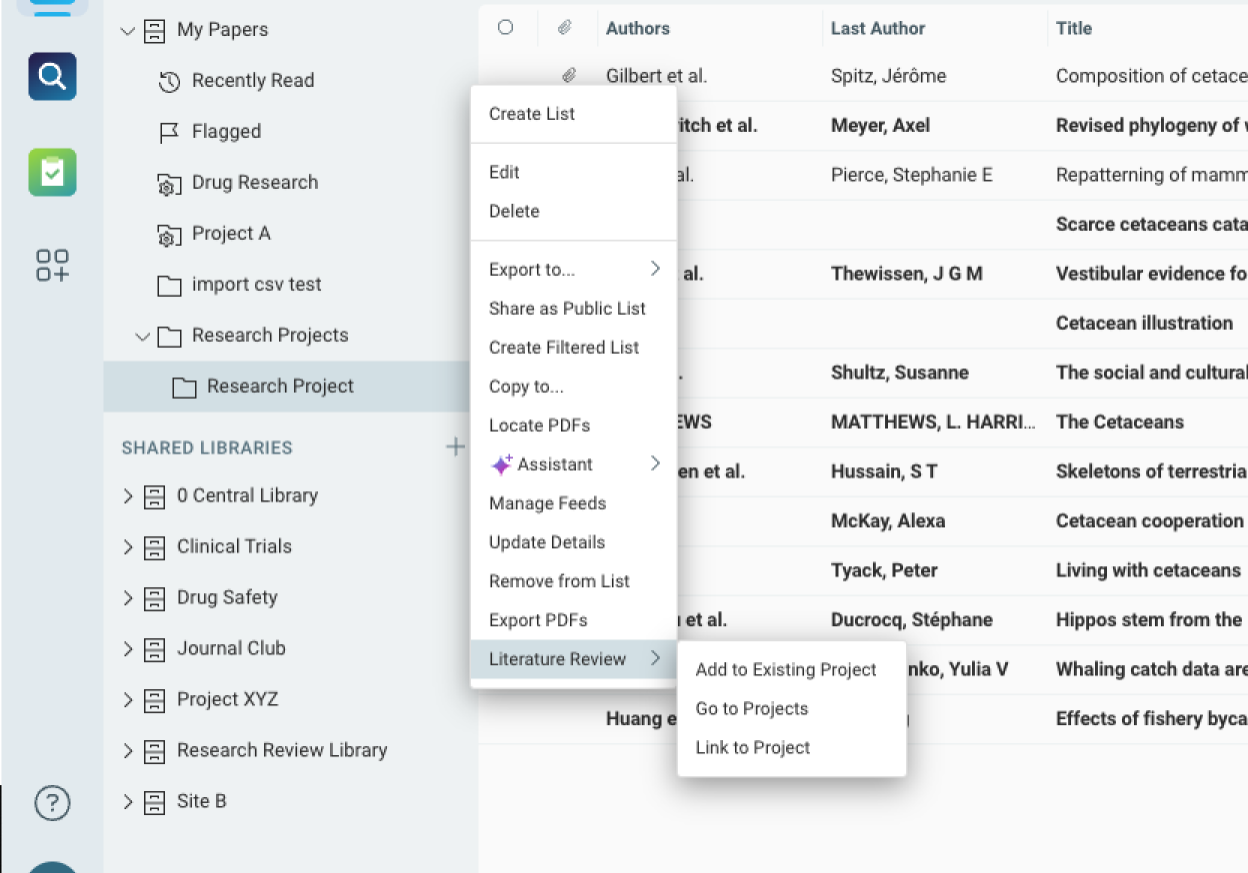
Cross-Functional Collaboration and Knowledge Sharing
Facilitate collaboration across medical, clinical, and regulatory teams with shared libraries and insights.
- Share key findings and annotated literature with teams to ensure alignment in decision-making.
- Enable collaboration between Medical Affairs, clinical development, and regulatory functions, ensuring all teams have visibility into relevant data.
Responsible for staying informed on new clinical trial data and regulatory changes across a variety of therapeutic areas?
Using ReadCube, a Medical Affairs team sets up custom alerts to monitor key therapeutic areas and new clinical studies. Literature is organized by drug class and indication, allowing the team to quickly retrieve relevant information when preparing reports or presentations.
By tagging important regulatory updates and clinical findings, they ensure compliance with the latest standards and can provide accurate, up-to-date data for medical decision-making. The result is faster, more informed responses to regulatory inquiries and internal strategy decisions.