ReadCube for Government Regulatory Agencies
Enhancing Literature Review and Monitoring for Regulatory Compliance
Regulatory agencies face the ongoing challenge of staying on top of vast amounts of scientific literature and reports. ReadCube’s end-to-end solution helps regulatory bodies manage and monitor critical research, ensuring they stay aligned with global regulatory standards and new scientific findings.
Key Features for
Regulatory Agencies
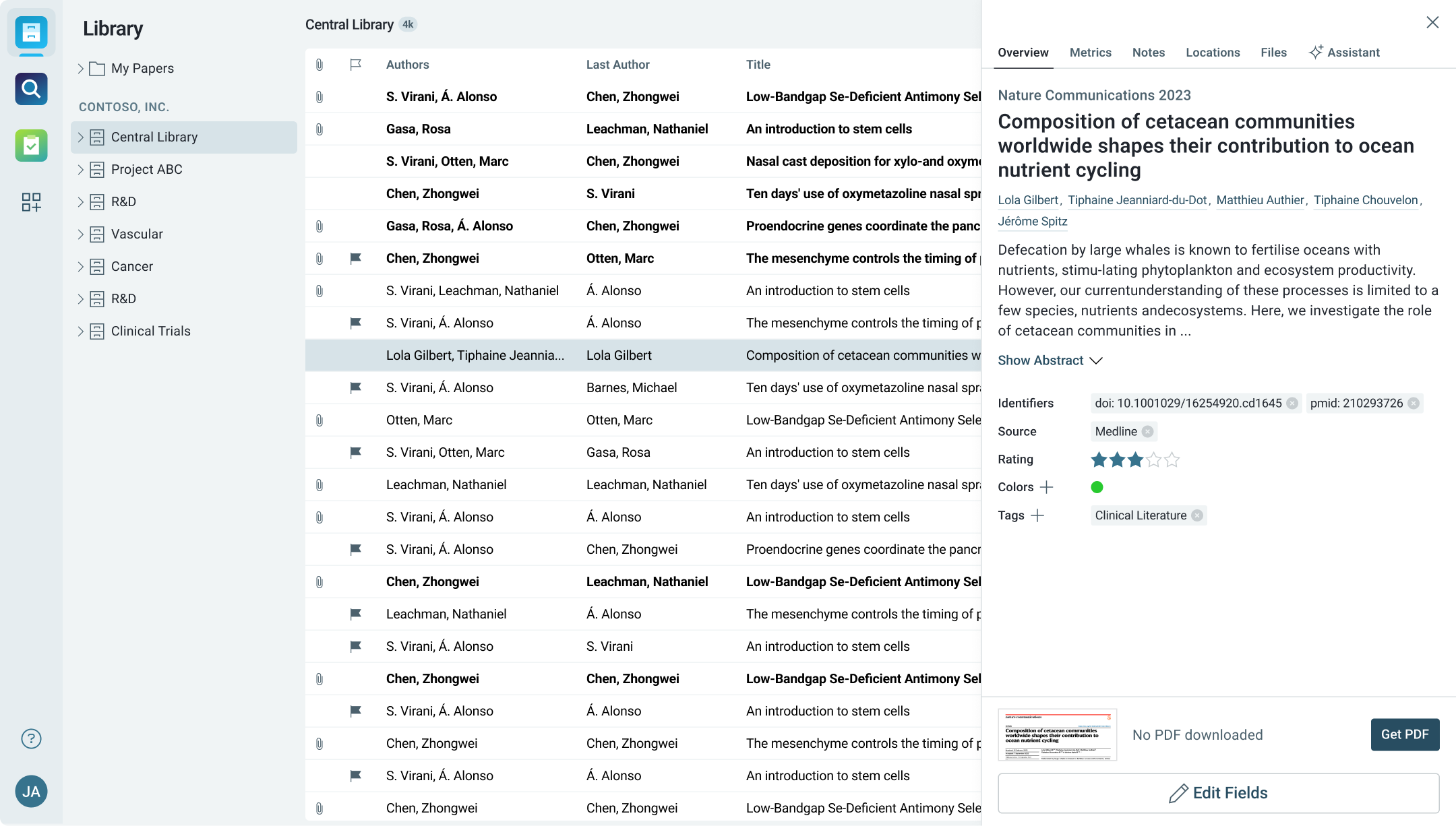
Comprehensive Literature Organization for Compliance and Auditing
Organize extensive research libraries, critical reports, and policy documents in a single, accessible space.
- Maintain structured libraries of regulatory reports, safety documents, and compliance papers.
- Categorize literature by area of regulation—health, environmental, consumer safety—and quickly locate relevant information during audits and reviews.
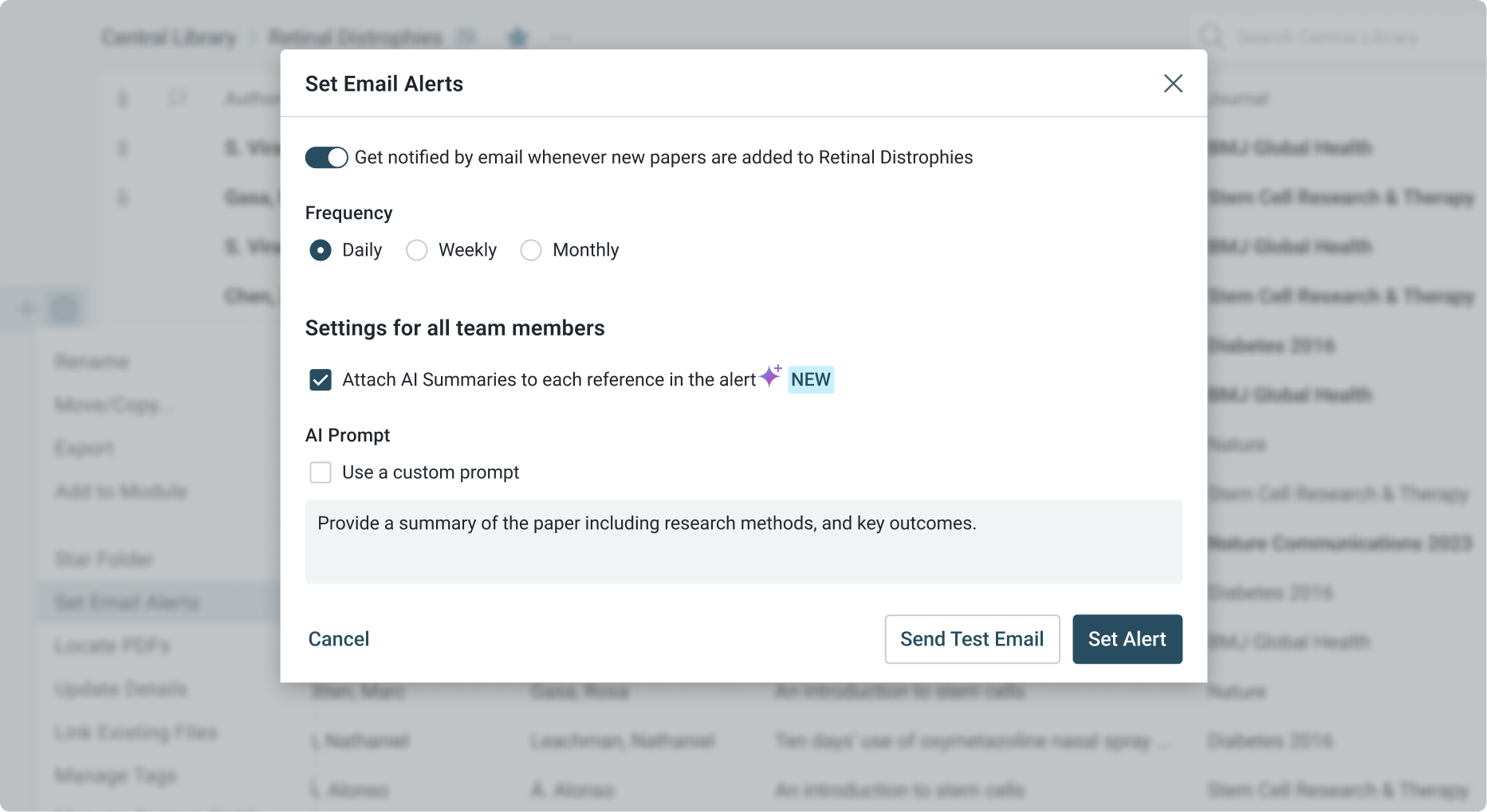
Continuous Monitoring of Regulatory Literature and Guidelines
Stay up to date with the latest research, scientific findings, and policy-relevant studies through ReadCube’s automated monitoring.
- Set up custom alerts to track updates to international regulations, industry standards, and safety guidelines.
- Monitor global publications related to regulatory developments, ensuring your agency remains up to date with new safety measures and best practices.
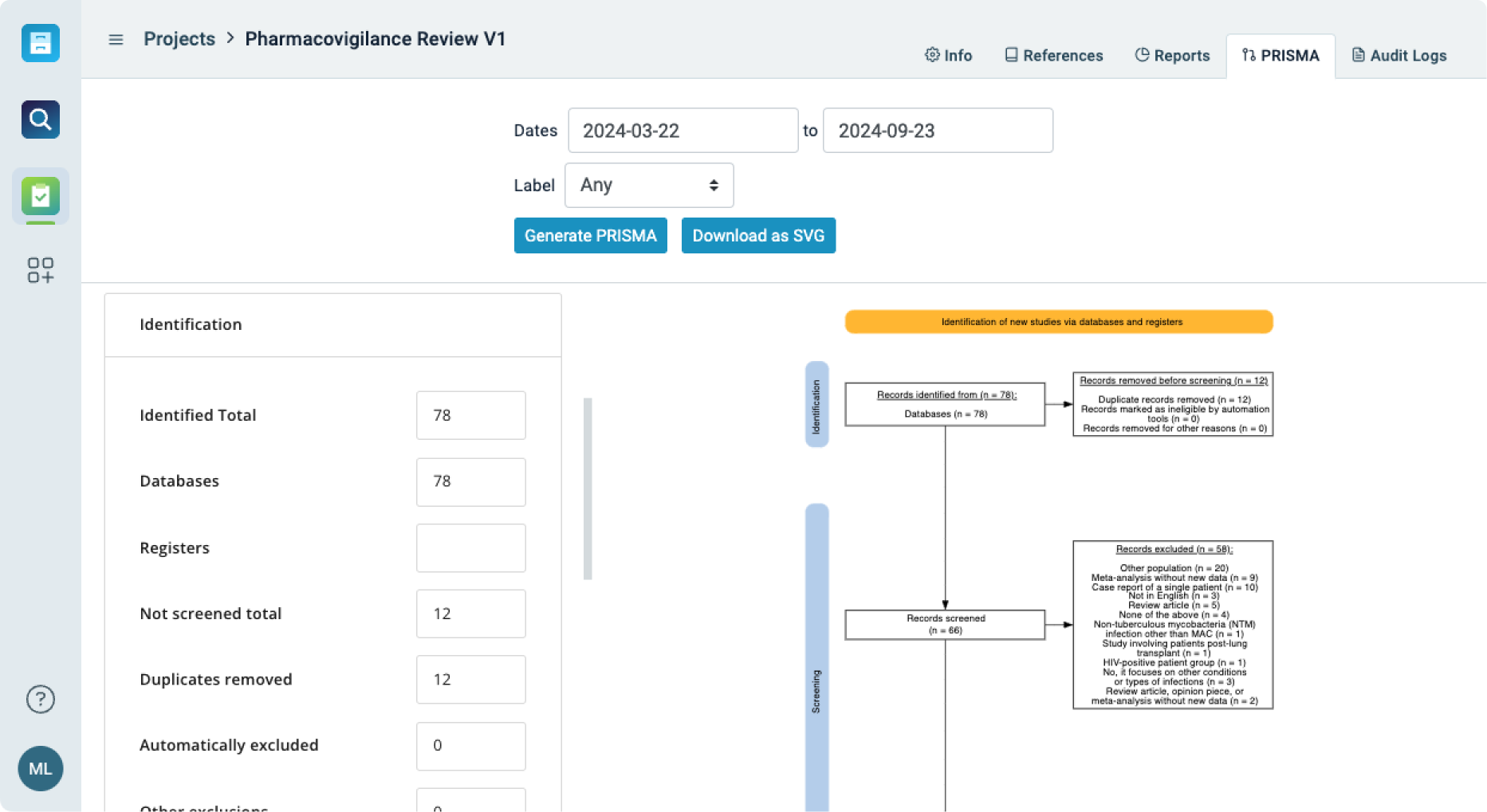
Streamlined Review Process for Regulatory Assessments
Simplify the process of conducting systematic reviews for policy evaluation, regulatory assessments, and evidence-based decision-making.
- Simplify the literature review process with workflows that manage the systematic evaluation of scientific data and regulatory guidelines.
- Conduct and document thorough assessments of safety reports, drug trials, environmental studies, and consumer protection analyses.