-
PDF
- Split View
-
Views
-
Cite
Cite
Rita Balice-Gordon, Garry D Honey, Christopher Chatham, Estibaliz Arce, Sridhar Duvvuri, Melissa Graham Naylor, Wenlei Liu, Zhiyong Xie, Nicholas DeMartinis, Brian T Harel, Gabriel H Braley, Rouba Kozak, Lovingly Park, David L Gray, A Neurofunctional Domains Approach to Evaluate D1/D5 Dopamine Receptor Partial Agonism on Cognition and Motivation in Healthy Volunteers With Low Working Memory Capacity, International Journal of Neuropsychopharmacology, Volume 23, Issue 5, May 2020, Pages 287–299, https://doi.org/10.1093/ijnp/pyaa007
Close - Share Icon Share
Abstract
Dopamine D1 receptor signaling plays key roles in core domains of neural function, including cognition and reward processing; however, many questions remain about the functions of circuits modulated by dopamine D1 receptor, largely because clinically viable, selective agonists have yet to be tested in humans.
Using a novel, exploratory neurofunctional domains study design, we assessed the safety, tolerability, pharmacodynamics, and pharmacokinetics of PF-06412562, a selective D1/D5R partial agonist, in healthy male volunteers who met prespecified criteria for low working memory capacity. Functional magnetic resonance imaging, electrophysiologic endpoints, and behavioral paradigms were used to assess working memory, executive function, and motivation/reward processing following multiple-dose administration of PF-06412562. A total of 77 patients were assigned PF-06412562 (3 mg twice daily and 15 mg twice daily) or placebo administered for 5 to 7 days. Due to the exploratory nature of the study, it was neither powered for any specific treatment effect nor corrected for multiple comparisons.
Nominally significant improvements from baseline in cognitive endpoints were observed in all 3 groups; however, improvements in PF-06412562–treated patients were less than in placebo-treated participants. Motivation/reward processing endpoints were variable. PF-06412562 was safe and well tolerated, with no serious adverse events, severe adverse events, or adverse events leading to dose reduction or temporary discontinuation except for 1 permanent discontinuation due to increased orthostatic heart rate.
PF-06412562, in the dose range and patient population explored in this study, did not improve cognitive function or motivation/reward processing more than placebo over the 5- to 7-day treatment period.
NCT02306876
The neurotransmitter dopamine modulates many brain functions. Signaling through the dopamine D1 receptor (D1R) affects cognition as well as motivation and reward processing. In some disorders, D1R signaling pathways are disrupted, and this disruption is thought to contribute to disease symptoms. It has been proposed that drugs that activate the dopamine D1R could be used to help patients with such conditions. This study evaluated a new drug that activates the dopamine D1R to assess the safety, and explore whether it affects brain functions associated with dopamine D1 signaling in a selected group of healthy volunteers. The results show that although the drug was safe, its effect on the brain functions associated with dopamine D1 signaling was no better than the effects of an inactive placebo.
Introduction
D1 dopamine receptors modulate many domains of neural function, including cognition, motivation, and motor control. Impairment in D1 receptor signaling contributes to cognitive impairment associated with schizophrenia, major depressive disorder, and Parkinson’s disease, among others (Lynch, 1992; Okubo et al., 1997a,, 1997b; Müller et al., 1998). There is therefore a strong clinical rationale to investigate new therapeutics that modulate D1 receptors for the treatment of psychiatric and neurological conditions (Arnsten et al., 1994; Goldman-Rakic et al., 2004; Takahashi et al., 2008,, 2012). Despite directed discovery efforts spanning nearly 40 years, there has been no viable agent available clinically to test the role of sustained D1 receptor activation in core domains of neural function. This is due in part to undesirable pharmacokinetics (PK) imparted by a catechol structure in all known selective agonists, which have complicated clinical assessments of D1 receptor function in healthy individuals and patients (Giardina and Williams, 2001; Taskinen et al., 2003; Buchanan et al., 2007; Meanwell, 2011).
PF-06412562 is an orally bioavailable partial agonist of D1 and D5 receptors (hereafter D1R) with a unique noncatechol structure and high selectivity over D2-like receptors. For in vitro assays, PF-06412562 stimulates cAMP production with an EC50 of 580 nM and an intrinsic activity of 44% relative to dopamine. Phase 1 single- and multiple-dose safety studies were conducted in healthy volunteers (Pfizer, data on file). A single-dose study of safety and pharmacodynamics (PD) completed in patients with Parkinson’s disease demonstrated that PF-06412562 elicits robust, centrally mediated pharmacology for 12 hours (Papapetropoulos et al., 2018). Preclinical toxicology studies support human dosing of up to 28 days in duration at exposures predicted to achieve clinically meaningful D1R occupancy in the brain. Thus, PF-06412562 is the first orally bioavailable, selective D1R partial agonist to be studied in the clinic.
Cognitive impairment and motivational deficits are believed to be, at least in part, the result of dysfunction in D1R-rich neural circuits that are common across several psychiatric and neurodegenerative illnesses. Several functional magnetic resonance imaging (fMRI) and electrophysiological measurements of brain function probing attention, motivation, working memory, and executive functions in healthy individuals and patients (Gur and Gur, 2010; Kariofillis et al., 2014) strongly support the involvement of mesocortical and mesolimbic dopaminergic networks in these functional domains. Furthermore, alterations of these networks are thought to be causally important for cognitive and motivational dysfunction (McNab and Klingberg, 2008; Cools et al., 2009; Gruber et al., 2014; Bolkan et al., 2016).
Given the potential for cognitive enhancement via dopamine and norepinephrine modulation, substantial effort has been devoted to investigating the effects of stimulant medications, particularly amphetamine and methylphenidate, on the domains of cognition and motivation in healthy individuals as well as patients (c.f. Marraccini et al., 2016). Collectively, the results of these studies suggest that a dopamine modulation strategy holds promise for cognitive enhancement (Arce and Ehlers, 2017). Results also clearly indicate the challenges associated with measuring the impact of pharmacological intervention on cognition and the need for more generalizable domain-based approaches to cognitive assessment (Smith and Farah, 2011; Marraccini et al., 2016).
Here we report a clinical study that explored the effects of D1R partial agonism with PF-06412562 on cognition and motivation/reward processing in healthy volunteers. The clinical study incorporated a neurofunctional domains approach that represents a paradigm shift for early clinical drug development from a focus on a single disease to a broader exploration of domains of neural dysfunction relevant to modulation of a particular target +(e.g., D1Rs). The trial described here is one of the first to apply principles outlined by the National Institute of Mental Health Research Domain Criteria project to drug discovery, which proposed that studies that probe domains of neural function that cut across traditional (i.e., Diagnostic and Statistical Manual of Mental Disorders or ICD) disease classifications could have utility for therapeutic discovery and development for psychiatric diseases (Cuthbert and Insel, 2010, 2013). This approach has several advantages over conventional clinical study designs, including the incorporation of multimodal assessments selected to establish whether a candidate therapeutic has PD effect(s) on target-rich, disease-relevant neural circuitry. A 2-stage study design, including endpoint analyses and interpretations, was developed to be domain-specific and disease independent. Successful modulation of cognitive and/or motivation/reward domains with PF-06212562 would provide a strong rationale for further clinical development in disorders where functional improvement of these domains is expected to provide significant therapeutic impact (e.g., cognitive impairment associated with schizophrenia and major depressive disorder, among others). Finally, domain-specific assessments may be translatable across species, streamlining future drug discovery and development efforts.
Among candidate targets for the domains of cognition, D1Rs have been hypothesized to enhance working memory by supporting the active maintenance of information in the face of interference (Durstewitz et al., 2000; O’Reilly, 2006). In vitro and in vivo studies have shown that D1R activation may enhance working memory by reducing background interference, resulting in enhancement of signal to noise in information processing (Yousif et al., 2016).
D1Rs are also highly relevant to motivation/reward dysfunction in psychiatric disease (World Health Organization, 1992; American Psychiatric Association DSM-5 Task Force, 2013). One commonly held hypothesis is that this dysfunction is caused by imbalance in the processing of positive vs negative prediction errors subserved by D1-dominant “direct” and the D2-dominant “indirect” corticostriatal pathways, respectively (Gerfen, 1992; Frank et al., 2004; Frank, 2005; Hong and Hikosaka, 2011; Collins and Frank, 2014; Nakanishi et al., 2014; Morita and Kawaguchi, 2015).
The PK and selectivity of PF-06412562 allow assessment of the effects of selective enhancement of D1R signaling. This study incorporated numerous D1R endpoints to assess impact of PF-06412562 in healthy patients on D1R-relevant measures of cognition, reward processing, and motivation, including associative learning or anticipation or consummation of reward/punishment, and subjective evaluation/decision-making in relation to reward valence/magnitude. The measures were multimodal, utilizing fMRI and electrophysiological endpoint—including evoked-response potentials and several behavioral paradigms—and were administered over a 7-day inpatient stay. To our knowledge, no equally broad and multimodal assessment of cognition and motivation/reward domains has previously been used to assess hypothesized pharmacological effects on these domains. Thus, this study also provided the opportunity to investigate the feasibility and applicability of this trial design.
The study objectives were to explore whether and how PF-06412562 modulated D1R-relevant networks and affected behavior in healthy volunteers using a neurofunctional domains study design. Prior studies have often failed to demonstrate enhancement of cognition in healthy individuals (Smith and Farah, 2011; Marraccini et al., 2016). We reasoned that beneficial cognitive as well as motivation/reward-related effects of PF-06412562 might be more readily detected in individuals with low working memory capacity given prior evidence that low working memory capacity may influence individual response to dopaminergic pharmacological interventions (Gibbs and D’Esposito, 2006; Frank and O’Reilly, 2006; Cools et al., 2008; van der Schaaf et al., 2013). Thus, volunteers were assessed for working memory capacity, and those with scores at screening corresponding to the lower one-third of a normative sample were enrolled (Redick et al., 2012). The safety, tolerability, and PK of PF-06412562 administered as immediate release (IR) and modified release (MR) tablets were also assessed. An additional objective was to explore the utility of several promising new endpoints and modalities for assessing potential therapeutic effects of enhanced D1R signaling. We reasoned that if PF-06412562 was found to modulate dopaminergic networks in individuals with low working memory capacity, this would support further development of PF-06412562 in several psychiatric and neurological indications.
Methods
Study Design
This study was a randomized, participant and investigator-blind, placebo-controlled, parallel design clinical trial. The study was designed to comprise 2 analytical stages: hypothesis generation (Stage 1) followed by hypothesis testing (Stage 2) (Figure 1). Both stages included multiple endpoints (behavioral, electrophysiologic, and imaging). In Stage 1, 2 doses of PF-06412562 (3 mg twice daily [BID] and 15 mg BID) were compared against the placebo arm for each endpoint with no multiplicity adjustment. Stage 1 sample size was based on operational feasibility. Up to 5 comparisons that met Stage 1 decision criteria were then to be formally powered and treated as primary comparisons in Stage 2, in which multiple comparisons were to be adjusted and overall type I error rate controlled across all primary comparisons, with continuous study enrollment through both stages.
Study design: planned 2-stage approach. POM, proof of mechanism.
PF-06412562 3 mg BID and 15 mg BID dosed as MR tablets were selected for evaluation in this study, as these doses were expected to result in mean D1 occupancy levels in the ranges of 5–10% and 15–30%, respectively, based on in vitro estimates of PF-06412562 binding affinity. This dosing paradigm allowed for testing of the study hypothesis at 2 doses with minimal overlap in exposures and projected central D1 occupancy with a potential to understand the exposure/dose response in different cognitive and behavioral domains.
Study Population
Eligible individuals were healthy, right-handed males, aged 18–45 years inclusive, without evidence or history of clinically significant hematologic, renal, endocrine, pulmonary, gastrointestinal, cardiovascular, hepatic, neurologic, or allergic disease, or Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Axis I psychiatric disorders as determined by the Mini International Neuropsychiatric Interview. The automated operation span task was used to screen individuals based on working memory capacity scores (Redick et al., 2012). Redick and co-workers reported assessment of working memory capacity in approximately 6000 individuals, including percentile scores. Based on these data, scores of 29–55 inclusive were categorized as “low” capacity, corresponding to the 5th to 33rd percentile range in the sample of Redick et al. (2012). To ensure participants were adequately engaged during the distractor component of the task, a score of ≥75% correct responses was required for math questions. To ensure average intelligence and rule out gross cognitive impairment, individuals were administered the Weschler Test of Adult Reading (for which Full Scale IQ scores were derived and required to be ≥80) (Wechsler, 2001) and the Mini Mental State Examination (for which participants had to demonstrate a score ≥27) (Folstein et al., 1975), respectively. Participants were also required to have at least a high school diploma or equivalent to participate in the study. These parameters enabled the identification of a sample of the general population with lower than average working memory capacity while otherwise cognitively, psychiatrically, and physically healthy.
All participants provided written informed consent. The protocol was reviewed and approved by the institutional review board (Aspire IRB, LLC, CA, Santee) at the investigational center participating in the study. This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Conference on Harmonisation Good Clinical Practice Guidelines.
PF-06412562 Administration
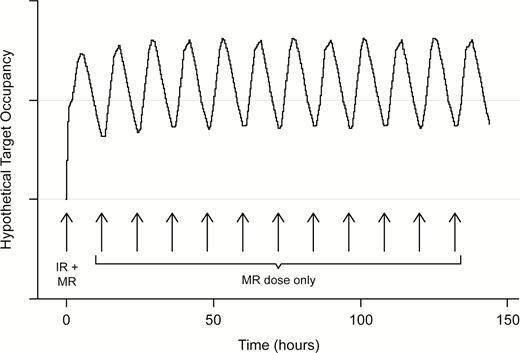
Doses of PF-06412562 (3 mg or 15 mg), administered as MR tablets or matched placebo, were given BID from day 1 through day 6. On day 7, only the morning dose was administered. The morning dose was administered at 8:00 am ±30 minutes, and the evening dose was approximately 12 hours after the morning dose. IR tablets were used as a loading dose on day 1 only to rapidly attain target drug concentrations required for testing. The first dose on day 1 consisted of IR tablets/placebo along with the MR tablets/placebo. Both doses (IR and MR tablets) were administered within 5 minutes of each other to facilitate rapid attainment of target concentrations (Figure 2). For participants assigned to the 3-mg BID group, one 3-mg MR tablet and one 1-mg PF-06412562 IR tablet were administered. For participants assigned to the 15-mg BID group, one 15-mg MR tablet and one 5-mg IR tablet were administered. Participants assigned to placebo received 1 MR placebo tablet and 1 IR placebo tablet. Subsequent doses were administered as only MR tablets at the assigned dose level. A follow-up visit for clinical safety assessments was conducted approximately 7 to 10 days following administration of the last dose. The Full Analysis Set (FAS) was defined as all participants randomized and who had received at least 1 dose of randomized treatment. The Per Protocol Analysis Set (PPAS) was a subset of the FAS dataset and included patients who received all doses of study treatment to which they were randomized, had no major protocol deviations, had a baseline measurement, and had at least 1 post-baseline measurement for at least 1 PD endpoint.
Hypothetical target occupancy profile achieved following the dosing paradigm. IR, immediate release; MR, modified release.
PK and Safety
PK and safety were assessed using standard measurements. Plasma samples were analyzed for PF-06412562 (parent) and PF-06663872 (metabolite) concentrations at WuXi AppTec (Shanghai, China) using a validated high-performance liquid chromatography tandem mass spectrometric method in compliance with sponsor standard operating procedures. Safety measurements included physical and neurological examinations, clinical laboratory measurements, electrocardiograms (ECGs), vital signs, and the recording of adverse events (AEs), applying Medical Dictionary for Regulatory Activities (version 19.0) coding.
PD
The PD, behavioral, electrophysiologic, and imaging assessments, summarized in Table 1, were endpoints selected to assess the effects of PF-06412562 on cognition and motivation/reward neurofunctional domains. Analyses of PD endpoints were conducted using the PPAS only.
Behavioral Batteries 1 and 2
| . | Tasks . |
|---|---|
| Behavioral battery 1 | Reading span task (Unsworth et al., 2009): reading span partial storage score |
| Change localization (Johnson et al., 2013): working memory capacity score, k | |
| Attentional capture task (ACT) (Fukuda and Vogel, 2011): capture cost = % accuracy of irrelevant flanker condition minus % accuracy of relevant flanker condition across stimulus-onset asynchronies | |
| Effort expenditure task (Treadway et al., 2009): proportion of high-effort choices during low-, medium-, and high-probability trials | |
| Working memory update/ignore task: total accuracy and reaction time for ignore trials, maintenance trials, and update trials; total accuracy and reaction time for ignore trials, update trials minus maintenance trials | |
| Digit symbol coding: total number of correct symbols | |
| Behavioral battery 2 | Symmetry span task (Redick et al., 2012): symmetry span partial storage score |
| Probabilistic selection task (Waltz et al., 2007): accuracy for choose A and avoid B conditions | |
| Attention network task (Fan et al., 2001): executive network efficiency, alerting efficiency; orienting efficiency | |
| Risk-based decision-making task (RBDM) (Norbury et al., 2013): proportion of choices of “experimental” over control gamble across conditions; proportion of times experimental gamble is selected when probability of winning is high vs low; proportion of times experimental gamble is selected when magnitude of possible gains is high vs low; proportion of times experimental gamble is selected when magnitude of possible losses is high vs low; reaction time for choosing “experimental” over control gamble across conditions; reaction time for choosing experimental gamble is selected when probability of winning is high vs low; reaction time for choosing experimental gamble is selected when magnitude of possible gains is high vs low | |
| Visual search task (Anderson et al., 2013): search efficiency (slope of reaction time by set size function) |
| . | Tasks . |
|---|---|
| Behavioral battery 1 | Reading span task (Unsworth et al., 2009): reading span partial storage score |
| Change localization (Johnson et al., 2013): working memory capacity score, k | |
| Attentional capture task (ACT) (Fukuda and Vogel, 2011): capture cost = % accuracy of irrelevant flanker condition minus % accuracy of relevant flanker condition across stimulus-onset asynchronies | |
| Effort expenditure task (Treadway et al., 2009): proportion of high-effort choices during low-, medium-, and high-probability trials | |
| Working memory update/ignore task: total accuracy and reaction time for ignore trials, maintenance trials, and update trials; total accuracy and reaction time for ignore trials, update trials minus maintenance trials | |
| Digit symbol coding: total number of correct symbols | |
| Behavioral battery 2 | Symmetry span task (Redick et al., 2012): symmetry span partial storage score |
| Probabilistic selection task (Waltz et al., 2007): accuracy for choose A and avoid B conditions | |
| Attention network task (Fan et al., 2001): executive network efficiency, alerting efficiency; orienting efficiency | |
| Risk-based decision-making task (RBDM) (Norbury et al., 2013): proportion of choices of “experimental” over control gamble across conditions; proportion of times experimental gamble is selected when probability of winning is high vs low; proportion of times experimental gamble is selected when magnitude of possible gains is high vs low; proportion of times experimental gamble is selected when magnitude of possible losses is high vs low; reaction time for choosing “experimental” over control gamble across conditions; reaction time for choosing experimental gamble is selected when probability of winning is high vs low; reaction time for choosing experimental gamble is selected when magnitude of possible gains is high vs low | |
| Visual search task (Anderson et al., 2013): search efficiency (slope of reaction time by set size function) |
Behavioral Batteries 1 and 2
| . | Tasks . |
|---|---|
| Behavioral battery 1 | Reading span task (Unsworth et al., 2009): reading span partial storage score |
| Change localization (Johnson et al., 2013): working memory capacity score, k | |
| Attentional capture task (ACT) (Fukuda and Vogel, 2011): capture cost = % accuracy of irrelevant flanker condition minus % accuracy of relevant flanker condition across stimulus-onset asynchronies | |
| Effort expenditure task (Treadway et al., 2009): proportion of high-effort choices during low-, medium-, and high-probability trials | |
| Working memory update/ignore task: total accuracy and reaction time for ignore trials, maintenance trials, and update trials; total accuracy and reaction time for ignore trials, update trials minus maintenance trials | |
| Digit symbol coding: total number of correct symbols | |
| Behavioral battery 2 | Symmetry span task (Redick et al., 2012): symmetry span partial storage score |
| Probabilistic selection task (Waltz et al., 2007): accuracy for choose A and avoid B conditions | |
| Attention network task (Fan et al., 2001): executive network efficiency, alerting efficiency; orienting efficiency | |
| Risk-based decision-making task (RBDM) (Norbury et al., 2013): proportion of choices of “experimental” over control gamble across conditions; proportion of times experimental gamble is selected when probability of winning is high vs low; proportion of times experimental gamble is selected when magnitude of possible gains is high vs low; proportion of times experimental gamble is selected when magnitude of possible losses is high vs low; reaction time for choosing “experimental” over control gamble across conditions; reaction time for choosing experimental gamble is selected when probability of winning is high vs low; reaction time for choosing experimental gamble is selected when magnitude of possible gains is high vs low | |
| Visual search task (Anderson et al., 2013): search efficiency (slope of reaction time by set size function) |
| . | Tasks . |
|---|---|
| Behavioral battery 1 | Reading span task (Unsworth et al., 2009): reading span partial storage score |
| Change localization (Johnson et al., 2013): working memory capacity score, k | |
| Attentional capture task (ACT) (Fukuda and Vogel, 2011): capture cost = % accuracy of irrelevant flanker condition minus % accuracy of relevant flanker condition across stimulus-onset asynchronies | |
| Effort expenditure task (Treadway et al., 2009): proportion of high-effort choices during low-, medium-, and high-probability trials | |
| Working memory update/ignore task: total accuracy and reaction time for ignore trials, maintenance trials, and update trials; total accuracy and reaction time for ignore trials, update trials minus maintenance trials | |
| Digit symbol coding: total number of correct symbols | |
| Behavioral battery 2 | Symmetry span task (Redick et al., 2012): symmetry span partial storage score |
| Probabilistic selection task (Waltz et al., 2007): accuracy for choose A and avoid B conditions | |
| Attention network task (Fan et al., 2001): executive network efficiency, alerting efficiency; orienting efficiency | |
| Risk-based decision-making task (RBDM) (Norbury et al., 2013): proportion of choices of “experimental” over control gamble across conditions; proportion of times experimental gamble is selected when probability of winning is high vs low; proportion of times experimental gamble is selected when magnitude of possible gains is high vs low; proportion of times experimental gamble is selected when magnitude of possible losses is high vs low; reaction time for choosing “experimental” over control gamble across conditions; reaction time for choosing experimental gamble is selected when probability of winning is high vs low; reaction time for choosing experimental gamble is selected when magnitude of possible gains is high vs low | |
| Visual search task (Anderson et al., 2013): search efficiency (slope of reaction time by set size function) |
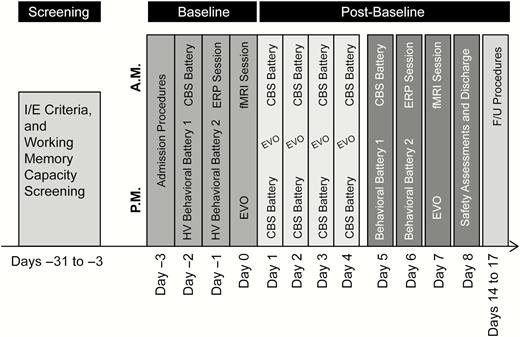
The Behavioral Batteries, Evoked-Response Potential (ERP) session, and fMRI session were performed during the baseline period and repeated once during the post-baseline period (Figure 3). The Behavioral Battery 1 was performed on day −2 and day 5. Behavioral Battery 2 and ERP Battery were performed on day −1 and day 6, and fMRI Battery was performed on day 0 and day 7. An additional battery, consisting of the Cambridge Brain Sciences (CBS) Battery and the Project: EVO™ tablet-based navigational video game task, was administered for the first time during the baseline period and then repeatedly on days 1 through 4 during the post-baseline period. On these days, participants completed 2 daily administrations of the CBS Battery and 1 daily administration of the EVO task. In addition, the CBS Battery was administered once during the morning of day 5 and the EVO task was administered during the afternoon of day 7 (Figure 3).
Assessment schedule. CBS, Cambridge Brain Sciences; fMRI, functional magnetic resonance imaging; F/U, follow-up; HV, healthy volunteer; I/E, inclusion/exclusion.
Behavioral Batteries 1 and 2
The administration of the behavioral tasks in Behavioral Batteries 1 and 2 was completed with a fixed order, for a maximum allocated time of 150 minutes, and with a 30-minute mandatory break. Details of the tasks included in the Behavioral Batteries are included in Table 1.
Neuroimaging/fMRI Battery
Participants completed anatomical and resting-state scans before the fMRI tasks. The fMRI task battery included 3 exploratory tasks: the Monetary Incentive Delay (MID) Task, N-back Task, and the AX-Continuous Performance Task (supplementary Table 1). A maximum time of 120 minutes was allowed for fMRI task battery completion. See Supplementary Materials and Methods for details of collection of MRI images, FMRI Expert Analysis Tool, and MID Task results.
ERP Battery
The ERP Battery included the contralateral delay activity task (Vogel and Machizawa, 2004; Vogel et al., 2005) and the implicit reward-biasing task (Santesso et al., 2009). The tasks and endpoints assessed are summarized in supplementary Table 2. See Supplementary Materials and Methods for details of the contralateral delay activity task and implicit reward-biasing task.
CBS Battery and EVO
The CBS Battery included composite scores for “short-term memory,” “reasoning,” and “verbal” domains. It comprised 12 tests that were self-administered in a fixed order covering 4 domains: (1) memory (spatial span, paired associates, digit span, spatial working memory); (2) planning (self-ordered search, spatial planning); (3) concentration (spatial rotation, interlocking polygons, feature matching); and (4) reasoning (deductive reasoning, verbal reasoning, and color–word remapping) (Hampshire et al., 2012).
EVO is a tablet-based navigation simulation game platform developed from an analogous task, “NeuroRacer” (Anguera et al., 2013), which incorporated a dual-tasking procedure. Participants were required to steer through a virtual environment while responding to peripheral targets that served as distractors. EVO testing included change from baseline in interference cost (ratio of performance on the targeting task [alone] to the targeting task during multi-tasking) in addition to performance on individual components.
Positive and Negative Affect Schedule—Expanded Form (PANAS-X)
The original PANAS is a 20-item self-report measure of positive (pleasurable symptoms) and negative affect (distress, unpleasurable engagement) developed by Watson and colleagues (Watson et al., 1988). The expanded version (PANAS-X), a 60-item self-report measure (Watson and Clark, 1994), includes the original 2 general factors (positive affect and negative affect) as well an additional 11 specific affects: fear, sadness, guilt, hostility, shyness, fatigue, surprise, joviality, self-assurance, attentiveness, and serenity. PANAS-X is a self-reported measure of positive and negative affect that allows the selection of only those subscales most relevant to the research. For the purposes of this study, 24 items were selected related to positive and negative affect as well as fatigue.
Statistical Analyses
In Stage 1, the treatment effect of each dose of PF-06412562 was tested against placebo for each exploratory PD endpoint (including Behavioral Battery, CBS Battery, EVO, ERP Battery, fMRI, composite scores, and PANAS-X endpoints). No multiple comparison adjustments were conducted for the Stage 1 analyses. All exploratory PD endpoints were presented as change from baseline measures.
The decision criteria for the primary analysis of the PD endpoints (specified by the sponsor prior to unblinding) were as follows: for comparison on a cognitive endpoint, 2-sided unadjusted P value for pairwise PF-06412562 vs placebo <.05 for each dose level; for comparison on a reward endpoint, 2-sided unadjusted P value for pairwise PF-06412562 vs placebo <.08 for each dose level; for comparison on a PANAS-X endpoint, 2-sided unadjusted P value for pairwise PF-06412562 vs placebo <.08 for each dose level. Different thresholds were used for cognitive and reward endpoints to account for a different number of endpoints in these pools so that about the same numbers of positive results would be expected by random chance.
Pearson correlation matrices for the baseline, post-baseline, and change from baseline values among different PD endpoints were reported. For CBS, EVO, and PANAS-X endpoints, see Supplementary Materials and Methods for more statistical details. ECG and vital sign data were summarized descriptively by treatment, nominal time post-dose, and day.
Results
The study was terminated following Stage 1 (hypothesis generation) and did not continue to Stage 2 based on the findings discussed below. Stage 1 data were combined with the data collected from participants who continued to be enrolled during the Stage 1 analysis for the final analyses presented here. All 77 participants who were enrolled in the study were male and were analyzed for AEs throughout study participation (FAS). One placebo participant discontinued early due to increased heart rate and orthostatic hypotension. In total, 76 of 77 participants who completed the study were analyzed for PD (PPAS) and laboratory data (Table 2). The mean participant age was 31.8 years (range 18–45 years), the mean weight was 80.8 kg (range 54.9–108.5 kg), and the mean body mass index was 26.0 kg/m2 (range 19.0–34.3 kg/m2). Demographic characteristics were similar across treatment groups.
Participant Disposition
| Participants, n . | PF-06412562 3 mg BID . | PF-06412562 15 mg BID . | Placebo . |
|---|---|---|---|
| Assigned to study treatment = 77 | |||
| Treated | 27 | 27 | 23 |
| Completed | 27 | 27 | 22 |
| Discontinued | 0 | 0 | 1 |
| AE | 0 | 0 | 1 |
| Analyzed for PK | |||
| Concentration | 27 | 27 | 0 |
| Analyzed for PD | |||
| Per Protocol Analysis Set | 27 | 27 | 22 |
| Analyzed for safety | |||
| AEs | 27 | 27 | 23 |
| Laboratory data | 27 | 27 | 22 |
| Participants, n . | PF-06412562 3 mg BID . | PF-06412562 15 mg BID . | Placebo . |
|---|---|---|---|
| Assigned to study treatment = 77 | |||
| Treated | 27 | 27 | 23 |
| Completed | 27 | 27 | 22 |
| Discontinued | 0 | 0 | 1 |
| AE | 0 | 0 | 1 |
| Analyzed for PK | |||
| Concentration | 27 | 27 | 0 |
| Analyzed for PD | |||
| Per Protocol Analysis Set | 27 | 27 | 22 |
| Analyzed for safety | |||
| AEs | 27 | 27 | 23 |
| Laboratory data | 27 | 27 | 22 |
Abbreviations: AE, adverse event; BID, twice daily; PD, pharmacodynamics; PK, pharmacokinetics.
Discontinuations had been attributed to the last study treatment received.
Participant Disposition
| Participants, n . | PF-06412562 3 mg BID . | PF-06412562 15 mg BID . | Placebo . |
|---|---|---|---|
| Assigned to study treatment = 77 | |||
| Treated | 27 | 27 | 23 |
| Completed | 27 | 27 | 22 |
| Discontinued | 0 | 0 | 1 |
| AE | 0 | 0 | 1 |
| Analyzed for PK | |||
| Concentration | 27 | 27 | 0 |
| Analyzed for PD | |||
| Per Protocol Analysis Set | 27 | 27 | 22 |
| Analyzed for safety | |||
| AEs | 27 | 27 | 23 |
| Laboratory data | 27 | 27 | 22 |
| Participants, n . | PF-06412562 3 mg BID . | PF-06412562 15 mg BID . | Placebo . |
|---|---|---|---|
| Assigned to study treatment = 77 | |||
| Treated | 27 | 27 | 23 |
| Completed | 27 | 27 | 22 |
| Discontinued | 0 | 0 | 1 |
| AE | 0 | 0 | 1 |
| Analyzed for PK | |||
| Concentration | 27 | 27 | 0 |
| Analyzed for PD | |||
| Per Protocol Analysis Set | 27 | 27 | 22 |
| Analyzed for safety | |||
| AEs | 27 | 27 | 23 |
| Laboratory data | 27 | 27 | 22 |
Abbreviations: AE, adverse event; BID, twice daily; PD, pharmacodynamics; PK, pharmacokinetics.
Discontinuations had been attributed to the last study treatment received.
PK
Steady-state plasma PF-06412562 concentrations following BID dosing of 3 mg (low dose) and 15 mg (high dose) as MR tablets were in expected ranges, and increases in PF-06412562 exposures were approximately dose proportional (Supplementary Table 3). Plasma PF-06663872 metabolite concentrations were approximately 8- to 10-fold lower than parent drug, as expected, and the increase in PF-06663872 exposure was approximately dose proportional between the 2 doses. Based on in vitro binding (Papapetropoulos et al., 2018), preclinical brain penetration estimates (data not shown), measured plasma exposures, and incorporating the partial agonism estimates for PF-06412562 and PF-06663872, these concentrations of parent plus metabolite are anticipated to result in relatively stable D1R activation in brain, averaging approximately 2.5% and 10% at the low and high doses, respectively.
PD Assessments: Behavioral Batteries 1 and 2
Behavioral Battery 1
No significant differences among the drug and placebo treatment groups were observed for any of the endpoints in the reading span task or change localization task. Thus, neither of these tasks met the study Stage 1 decision criteria. The attentional capture task had 1 endpoint that met the Stage 1 decision criteria (P < .05) for both doses vs placebo comparisons: capture cost with stimulus-onset asynchrony was 250 ms. Both drug groups demonstrated a decrease in capture cost over the treatment period of the study, while the placebo group demonstrated an increase in capture cost (Table 3). A decrease in capture cost, defined as the difference in accuracy between irrelevant and relevant flanker conditions, is thought to indicate an improvement in attentional control (Fukuda & Vogel, 2011). However, the decrease in capture cost in both dose groups did not reflect improved performance in either condition as the unadjusted mean accuracies (data not shown) increase from baseline to post-baseline in both conditions (irrelevant vs relevant flanker) for the placebo group. This suggests that the capture cost variable in the task as administered in this study did not differentiate processing relevant from irrelevant stimuli as intended. In the 15-mg dose group, the unadjusted mean accuracies decreased from baseline to post-baseline. None of the endpoints in the effort expenditure task or the working memory update/ignore task showed significant differences among the drug and placebo treatment groups. For the digit symbol coding task, 1 endpoint, total number of correct symbols, met the Stage 1 decision criteria (P < .05) for both PF-06412562 doses vs placebo comparisons. All treatment groups improved from baseline (LS means > 0); however, the placebo group had the greatest LS mean change from baseline (Supplementary Table 4).
Statistical Summary (RM-ANCOVA) of Change From Baseline in Capture Cost in Attentional Capture Task
| . | . | . | . | . | Contrast of PF-06412562 vs placebo . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| Stimulus onset asynchrony . | Treatment . | n . | LS means . | SE . | LS means . | SE . | 95% CI range . | P value . |
| 50 ms | PF-06412562 | 27 | 0.011 | 0.025 | 0.011 | 0.039 | (−0.065, 0.088) | .7739 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | 0.020 | 0.026 | 0.021 | 0.040 | (−0.057, 0.099) | .5999 | |
| 15 mg BID | ||||||||
| Placebo | 22 | −0.001 | 0.030 | |||||
| 150 ms | PF-06412562 | 27 | 0.045 | 0.025 | 0.041 | 0.039 | (−0.036, 0.117) | .2945 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.048 | 0.026 | −0.052 | 0.039 | (−0.129, 0.025) | .1871 | |
| 15 mg BID | ||||||||
| Placebo | 22 | 0.004 | 0.030 | |||||
| 250 ms | PF-06412562 | 27 | −0.047 | 0.025 | −0.086 | 0.040 | (−0.164, −0.008) | .0311 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.076 | 0.027 | −0.115 | 0.041 | (−0.196, −0.034) | .0053 | |
| 15 mg BID | ||||||||
| Placebo | 22 | 0.039 | 0.031 | |||||
| 350 ms | PF-06412562 | 27 | −0.015 | 0.025 | 0.050 | 0.039 | (−0.027, 0.128) | .2013 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.050 | 0.027 | 0.015 | 0.041 | (−0.064, 0.095) | .7099 | |
| 15 mg BID | ||||||||
| Placebo | 22 | −0.065 | 0.030 |
| . | . | . | . | . | Contrast of PF-06412562 vs placebo . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| Stimulus onset asynchrony . | Treatment . | n . | LS means . | SE . | LS means . | SE . | 95% CI range . | P value . |
| 50 ms | PF-06412562 | 27 | 0.011 | 0.025 | 0.011 | 0.039 | (−0.065, 0.088) | .7739 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | 0.020 | 0.026 | 0.021 | 0.040 | (−0.057, 0.099) | .5999 | |
| 15 mg BID | ||||||||
| Placebo | 22 | −0.001 | 0.030 | |||||
| 150 ms | PF-06412562 | 27 | 0.045 | 0.025 | 0.041 | 0.039 | (−0.036, 0.117) | .2945 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.048 | 0.026 | −0.052 | 0.039 | (−0.129, 0.025) | .1871 | |
| 15 mg BID | ||||||||
| Placebo | 22 | 0.004 | 0.030 | |||||
| 250 ms | PF-06412562 | 27 | −0.047 | 0.025 | −0.086 | 0.040 | (−0.164, −0.008) | .0311 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.076 | 0.027 | −0.115 | 0.041 | (−0.196, −0.034) | .0053 | |
| 15 mg BID | ||||||||
| Placebo | 22 | 0.039 | 0.031 | |||||
| 350 ms | PF-06412562 | 27 | −0.015 | 0.025 | 0.050 | 0.039 | (−0.027, 0.128) | .2013 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.050 | 0.027 | 0.015 | 0.041 | (−0.064, 0.095) | .7099 | |
| 15 mg BID | ||||||||
| Placebo | 22 | −0.065 | 0.030 |
Abbreviations: BID, twice daily; CI, confidence interval; LS, least squares; RM-ANCOVA, repeated measure analysis of covariance.
Statistical Summary (RM-ANCOVA) of Change From Baseline in Capture Cost in Attentional Capture Task
| . | . | . | . | . | Contrast of PF-06412562 vs placebo . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| Stimulus onset asynchrony . | Treatment . | n . | LS means . | SE . | LS means . | SE . | 95% CI range . | P value . |
| 50 ms | PF-06412562 | 27 | 0.011 | 0.025 | 0.011 | 0.039 | (−0.065, 0.088) | .7739 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | 0.020 | 0.026 | 0.021 | 0.040 | (−0.057, 0.099) | .5999 | |
| 15 mg BID | ||||||||
| Placebo | 22 | −0.001 | 0.030 | |||||
| 150 ms | PF-06412562 | 27 | 0.045 | 0.025 | 0.041 | 0.039 | (−0.036, 0.117) | .2945 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.048 | 0.026 | −0.052 | 0.039 | (−0.129, 0.025) | .1871 | |
| 15 mg BID | ||||||||
| Placebo | 22 | 0.004 | 0.030 | |||||
| 250 ms | PF-06412562 | 27 | −0.047 | 0.025 | −0.086 | 0.040 | (−0.164, −0.008) | .0311 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.076 | 0.027 | −0.115 | 0.041 | (−0.196, −0.034) | .0053 | |
| 15 mg BID | ||||||||
| Placebo | 22 | 0.039 | 0.031 | |||||
| 350 ms | PF-06412562 | 27 | −0.015 | 0.025 | 0.050 | 0.039 | (−0.027, 0.128) | .2013 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.050 | 0.027 | 0.015 | 0.041 | (−0.064, 0.095) | .7099 | |
| 15 mg BID | ||||||||
| Placebo | 22 | −0.065 | 0.030 |
| . | . | . | . | . | Contrast of PF-06412562 vs placebo . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| Stimulus onset asynchrony . | Treatment . | n . | LS means . | SE . | LS means . | SE . | 95% CI range . | P value . |
| 50 ms | PF-06412562 | 27 | 0.011 | 0.025 | 0.011 | 0.039 | (−0.065, 0.088) | .7739 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | 0.020 | 0.026 | 0.021 | 0.040 | (−0.057, 0.099) | .5999 | |
| 15 mg BID | ||||||||
| Placebo | 22 | −0.001 | 0.030 | |||||
| 150 ms | PF-06412562 | 27 | 0.045 | 0.025 | 0.041 | 0.039 | (−0.036, 0.117) | .2945 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.048 | 0.026 | −0.052 | 0.039 | (−0.129, 0.025) | .1871 | |
| 15 mg BID | ||||||||
| Placebo | 22 | 0.004 | 0.030 | |||||
| 250 ms | PF-06412562 | 27 | −0.047 | 0.025 | −0.086 | 0.040 | (−0.164, −0.008) | .0311 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.076 | 0.027 | −0.115 | 0.041 | (−0.196, −0.034) | .0053 | |
| 15 mg BID | ||||||||
| Placebo | 22 | 0.039 | 0.031 | |||||
| 350 ms | PF-06412562 | 27 | −0.015 | 0.025 | 0.050 | 0.039 | (−0.027, 0.128) | .2013 |
| 3 mg BID | ||||||||
| PF-06412562 | 27 | −0.050 | 0.027 | 0.015 | 0.041 | (−0.064, 0.095) | .7099 | |
| 15 mg BID | ||||||||
| Placebo | 22 | −0.065 | 0.030 |
Abbreviations: BID, twice daily; CI, confidence interval; LS, least squares; RM-ANCOVA, repeated measure analysis of covariance.
Behavioral Battery 2
No significant differences among the drug and placebo groups were observed for the symmetry span task, probabilistic selection task, and attention network test. Six of the risk-based decision-making task endpoints met the Stage 1 decision criteria (P < .08) (Supplementary Table 5). Five of these 6 effects pertained to differential reductions in median RT from baseline to follow-up in the 15-mg group compared with placebo. Specifically, median RT was more strongly reduced at follow-up in the 15-mg group for the following endpoints: median RT when magnitude of possible losses was 30; median RT when magnitude of possible gains was 30; median RT when magnitude of possible gains was 70; median RT when probability of winning was low; and median RT when probability of winning was high. Finally, compared with placebo, both dose groups also showed a smaller increase from baseline to follow-up in the influence of gain magnitude on risky choice (i.e., the proportion of experimental gambles when magnitude of possible gains was 70–30 endpoint).
No differences were observed among drug and placebo groups on any of the endpoints for the visual search task.
ERP Battery
No significant differences were observed among drug and placebo groups on any of the endpoints in the ERP Battery (the contralateral delay activity task or the implicit reward-biasing task).
Neuroimaging/fMRI Battery
Within the fMRI Battery, 5 MID Task endpoints met the Stage 1 decision criteria for the 3-mg vs placebo comparison. All these endpoints pertained to subcortical regions of interest (ROIs), with 3 of the 5 involving the contrast of gain > no gain cues during the anticipation phase of the task and the remaining 2 involving the contrast of hit loss > miss loss during the consummation phase. Specifically, relative to the placebo group, the 3-mg group showed an enhanced BOLD response in left accumbens and bilateral putamen during cues that signaled the availability vs nonavailability of reward alongside a decreased BOLD response in bilateral caudate during successful vs unsuccessful loss avoidance. For both contrasts and both hemispheres, similar but less pronounced effects were observed in the 15-mg group, though these effects within the 15-mg treatment group were not significant compared with placebo for these or any other MID Task endpoint (Supplementary Table 6). No significant differences were observed among drug and placebo groups on any of the endpoints for the N-back task or the AX-continuous performance task or for resting-state fMRI.
CBS Battery and EVO
For CBS Battery scores, 2 of the 3 composite scores met the Stage 1 decision criteria (P < .05) for both PF-06412562 doses vs placebo comparisons: composite short-term memory score and composite reasoning score. While all treatment groups improved from baseline, the placebo group had the greatest improvement from baseline at every time point compared with either dose of PF-06412562 (Supplementary Table 7). Two of the EVO endpoints met the Stage 1 decision criteria (P < .05) for the comparison of 15 mg vs placebo. These were the mean threshold single task (targeting) and the mean threshold averaged across single task and multi-task. An increase indicated an improvement in performance (Supplementary Table 8). In both endpoints, the placebo group increased more than the 15-mg PF-06412562 dose group. Further assessment of the data suggested that the effect on the mean threshold averaged across single task and multi-task was driven by the mean threshold averaged across single task, as there was no difference between groups for the multi-task condition that required processing a higher cognitive load.
PANAS-X
There were no significant differences among drug and placebo groups for any of the endpoints on the PANAS-X task.
Safety
All-causality and treatment-related treatment-emergent AEs (TEAEs) are summarized in Table 4. There were no deaths, serious AEs, severe AEs, or AEs leading to participant dose reduced or temporary discontinuation in this study, although 1 participant in the placebo group was permanently discontinued from the study due to AEs (orthostatic heart-rate response increased). In all treatment groups, most of the TEAEs were treatment related; PF-06412562 3 mg BID (17 out of 19), PF-06412562 15 mg BID (34 out of 34), and placebo (23 out of 25) groups. Overall, 1 TEAE (dizziness) and 3 TEAEs (orthostatic heart-rate response increased, orthostatic hypotension, and anxiety) of moderate severity (all treatment related) were reported by participants in the PF-06412562 3-mg BID and placebo treatment groups, respectively. All other AEs were mild in severity. The types of AEs that were reported with PF-06412562 were consistent with those seen earlier in the development program (Pfizer, data on file). The most commonly reported AEs were headache and nausea. The AE of headache was experienced by 6 participants (5 treatment related) in the PF-06412562 3-mg BID group, 8 participants (all treatment related) in the PF-06412562 15-mg BID group, and 5 participants (all treatment related) in the placebo group. The AE of nausea was experienced by 1 participant (treatment related) in the PF-06412562 3-mg BID group, 8 participants (all treatment related) in the PF-06412562 15-mg BID group, and 2 participants (both treatment related) in the placebo group. All other AEs were reported by 3 or fewer participants for any of the treatments. There were no clinically significant changes in vital signs, ECG, or laboratory abnormalities in any of the PF-06512562 treated groups.
Treatment-Emergent Adverse Events: All-Causality (Treatment Related)
| . | PF-064125623 mg BID . | PF-0641256215 mg BID . | Placebo . |
|---|---|---|---|
| Participants evaluable for AEs | 27 (27) | 27 (27) | 23 (23) |
| AEs, n | 19 (17) | 34 (34) | 25 (23) |
| Participants, n | |||
| With AEs | 14 (13) | 18 (18) | 12 (12) |
| With SAEs | 0 | 0 | 0 |
| With severe AEs | 0 | 0 | 0 |
| Discontinued due to AEs | 0 | 0 | 1 (1) |
| With dose reduced or temporary discontinuation due to AEs | 0 | 0 | 0 |
| . | PF-064125623 mg BID . | PF-0641256215 mg BID . | Placebo . |
|---|---|---|---|
| Participants evaluable for AEs | 27 (27) | 27 (27) | 23 (23) |
| AEs, n | 19 (17) | 34 (34) | 25 (23) |
| Participants, n | |||
| With AEs | 14 (13) | 18 (18) | 12 (12) |
| With SAEs | 0 | 0 | 0 |
| With severe AEs | 0 | 0 | 0 |
| Discontinued due to AEs | 0 | 0 | 1 (1) |
| With dose reduced or temporary discontinuation due to AEs | 0 | 0 | 0 |
Abbreviations: AE, adverse event; BID, twice daily; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
Except for the number of AEs, participants were counted only once per treatment in each row.
MedDRA (version 19.0) coding dictionary applied.
Treatment-Emergent Adverse Events: All-Causality (Treatment Related)
| . | PF-064125623 mg BID . | PF-0641256215 mg BID . | Placebo . |
|---|---|---|---|
| Participants evaluable for AEs | 27 (27) | 27 (27) | 23 (23) |
| AEs, n | 19 (17) | 34 (34) | 25 (23) |
| Participants, n | |||
| With AEs | 14 (13) | 18 (18) | 12 (12) |
| With SAEs | 0 | 0 | 0 |
| With severe AEs | 0 | 0 | 0 |
| Discontinued due to AEs | 0 | 0 | 1 (1) |
| With dose reduced or temporary discontinuation due to AEs | 0 | 0 | 0 |
| . | PF-064125623 mg BID . | PF-0641256215 mg BID . | Placebo . |
|---|---|---|---|
| Participants evaluable for AEs | 27 (27) | 27 (27) | 23 (23) |
| AEs, n | 19 (17) | 34 (34) | 25 (23) |
| Participants, n | |||
| With AEs | 14 (13) | 18 (18) | 12 (12) |
| With SAEs | 0 | 0 | 0 |
| With severe AEs | 0 | 0 | 0 |
| Discontinued due to AEs | 0 | 0 | 1 (1) |
| With dose reduced or temporary discontinuation due to AEs | 0 | 0 | 0 |
Abbreviations: AE, adverse event; BID, twice daily; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
Except for the number of AEs, participants were counted only once per treatment in each row.
MedDRA (version 19.0) coding dictionary applied.
Discussion
The study reported here was designed to test the hypothesis that enhanced D1R activation would improve cognition and motivation/reward processing in healthy individuals with low working memory capacity as a proxy for low baseline dopaminergic activity. The study utilized a neurofunctional domains approach that incorporated several multimodal assessments selected to determine whether PF-06412562 pharmacodynamically affected neural circuitry and domains of cognition and motivation/reward that are believed to be modulated by D1Rs. A 2-stage study design was used, with Stage 1 being hypothesis generating and Stage 2 intended to be hypothesis testing. Endpoints were selected based on the functional engagement of neurocircuitry known to involve dopaminergic innervation and/or be responsive to dopaminergic perturbation. We do not imply exclusive or even predominant dependence of these endpoints on dopamine function, nor is this a central assumption of the study. The endpoints provide a series of related assessments based on interrogation of different nodes within known circuitry rather than a comprehensive assessment of broad aspects of cognition. We note that there is little evidence to date that neurofunctional domains can be separately manipulated by relevant pharmacology.
Prior to dose selection, a structurally and pharmacologically similar noncatechol D1R partial agonist was evaluated in the rodent cTUNL working memory model (Talpos et al., 2010), and single doses that significantly improved task performance correlated to approximately 10% and 25% D1R occupancy in brain (Kozak et al., Society for Neuroscience, 2017). PF-06412562 reversed ketamine-induced deficits at 3 different single-dose levels in a primate delayed spatial response model of working memory (Kozak et al., Society for Neuroscience, 2017). Based on these results and those of other unpublished preclinical studies, we hypothesized that the enhancement of D1R signaling projected from 3-mg and 15-mg doses, respectively, would result in improvement in working memory, particularly in the protection of information from interference. Previous work suggests that there is an optimal range of dopaminergic enhancement of working memory that reflects baseline dopamine levels, and enhancement of D1 activity beyond this optimal range may reverse improvement given the inverted U-shaped relationship between cognition and prefrontal dopaminergic activity (Kimberg et al., 1997; van der Schaaf et al., 2013). Thus, we hypothesized that PF-06412562 could potentially improve aspects of cognition in the low working memory capacity group and might have no effect in high working memory–capacity participants. Furthermore, we were alert to the possibility of a nonlinear dose–response relationship, particularly at receptor occupancies above the range we targeted here in low working memory–capacity participants. However, treatment with PF-06412562 was not associated with any unambiguous improvements compared with placebo on tasks assessing cognition or motivation/reward domains in healthy male participants with low working memory capacity. For this reason and because many endpoints did not meet the criteria established for proceeding to Stage 2, the study was terminated after evaluation of the Stage 1 results. Thus, the findings reported here are descriptive and potentially hypothesis-generating only. For the few cognitive endpoints that did meet Stage 1 decision criteria, there was an inconsistent pattern of improved performance from baseline over the treatment period in all 3 groups, with the 3-mg and/or 15-mg groups improving less than the placebo group. For example, 1 or both the dose groups showed less improvement than placebo from baseline to follow-up in digit symbol coding, CBS, and EVO endpoints meeting Stage 1 decision criteria. These findings could be consistent with a potential inverted U-shaped dose response with this mechanism, but the target exposures for the dose groups in this study were not expected to lead to this type of response; this possibility remains to be explored further. One difference in this study vs the preclinical studies was that PF-06412562 was administered with continuous exposure, while all preclinical experiments demonstrating efficacy used single doses or once-daily intermittent exposure. However, this pattern of active vs placebo findings was not consistently observed across all tasks intended to assess similar domains of functioning. Nevertheless, testing the clinical hypothesis that extends directly from the preclinical cognition and reward/motivation assay data using acute and acute intermittent dosing has yet to be tested in the patients, and the continuous exposure approach used in this and a parallel study in schizophrenia (Arce et al., 2019) has yet to be examined in any of the preclinical assays that informed the design of these studies.
Similarly, the few endpoints relevant to motivation/reward that met Stage 1 decision criteria were also only inconsistently observed. For example, reaction times were generally faster during the risk-based decision-making task at follow-up, and this effect was stronger only for the 15-mg group relative to placebo. We note these changes cannot be clearly interpreted as a performance improvement, as they might arise from either enhanced task vigilance or from the adoption of less deliberative response strategies. The latter interpretation is potentially more consistent with the significantly reduced effect of gain magnitude at follow-up in the 15-mg group. Several endpoints from the MID Task also met Stage 1 decision criteria, although these effects were never found in behavioral performance metrics and were exclusively present in the 3-mg dose group. Thus, altogether, neither the observed cognitive nor reward/motivation effects meeting Stage 1 decision criteria could be unambiguously interpreted as improvements of performance due to treatment with PF-06412562, nor were these effects observed consistently across all doses or all tasks intended to assess the same neurofunctional domain.
One possibility is that the above findings meeting Stage 1 decision criteria are, in fact, false positives. Indeed, it was not possible to quantify the likelihood of the observed pattern of results under the global null hypothesis, because multiple comparisons were not corrected for in this exploratory study. It is also possible that these findings passing the Stage 1 decision criteria are indeed true effects and it is the corresponding null findings that are in error (i.e., they are false negatives). It is also possible that certain tasks are more sensitive than others to the effects of PF-06412562, possibly because they invoke somewhat different cognitive processes related to D1 receptor signaling or have psychometric properties that make the tests more suited for detecting change.
We note that it remains unclear whether a healthy volunteer population—even one with low working memory capacity—is an appropriate participant population to detect pro-cognitive or pro-motivation/reward effects of D1R modulation. Prior studies have demonstrated the impact of dopaminergic manipulations on reward/motivation and cognition and that these can depend on baseline working memory capacity (hypothesized to be a proxy for baseline dopamine levels; Kimberg et al., 1997; van der Schaaf et al., 2013). However, beneficial effects of dopamine modulation can be observed in these domains without stratifying by working memory capacity (Murphy et al., 2016). In the case of PF-06412562, the current study reveals no pro-cognitive effects of repeated dosing in healthy volunteers with low working memory. This is consistent with the absence of pro-cognitive effects in a separate 14-day, multiple-dose safety study (NCT01959594) with PF-06412562 doses of 9, 30, and 75 mg/d in healthy volunteers (data on file). These results contrast with a third study involving PF-06412562 administered as a single dose to an unenriched healthy volunteer population that did reveal relevant, dose-responsive effects on effort- and reward-based decision-making across single doses of 6, 15, and 30 mg (Soutschek et al., 2019). It is notable that this clinical experiment that successfully translated the preclinical experiments also used the same dosing paradigm in contrast to the current study. Given these discrepant outcomes and the lack of a direct 1:1 comparison between enriched and nonenriched study designs, the utility of the enrichment for low working memory capacity cannot be decisively evaluated.
Notwithstanding their low working memory capacity, the current study participants were nonetheless healthy volunteers; one may ask whether different or more consistent effects of PF-06412562 could conceivably be obtained in patient groups with low working memory capacity or who present with other functional deficits linked to low dopamineD1 tone in D1R-rich regions. Although modeling conducted prior to the study suggested low potential for functional D1R antagonism at the targeted receptor occupancies, and the results of Soutschek et al. with PF-06412562 support this assumption, the partial agonist pharmacology of this compound adds an additional nuance to the question of appropriate participant selection. We note that multiple studies in nonhuman primates have demonstrated single-dose pro-cognitive effects of D1R agonists in schizophrenia-related models with very low projected engagement at D1Rs (Cai et al., 1997; Castner et al., 2004); however, whether these results can be translated to a clinical effect remain unknown. In a companion study employing PF-06412562 and the same neurofunctional domains approach in patients with schizophrenia following the same continuous exposure dosing paradigm, no functional improvement in cognition or motivation/reward processing was observed (Arce et al., 2019). Similarly, intermittent low dosing of the full agonist DAR-0100A, a short-acting full D1R agonist, did not consistently improve cognition in individuals with schizophrenia (Girgis et al., 2016). These results highlight remaining unknowns and challenges with translating preclinical cognitive data, although further work with this mechanism using other D1R activators (including positive allosteric modulators), dosing paradigms, or patient selection approaches may provide additional important information.
In contrast to the relatively few investigations of D1R activation, approximately 200 published clinical studies have examined the effects of amphetamine or methylphenidate in healthy volunteers in the domain of cognition (Smith and Farah, 2011; Marraccini et al., 2016). While the results are mixed, when drug effects were assessed as a function of individual parameters—particularly placebo performance, genotype, or self-reported impulsivity—cognitive enhancement was observed to be greatest for participants who performed most poorly on placebo, had a catechol-O-methyltransferase genotype associated with poorer executive function, or self-reported being impulsive in their everyday lives (Marraccini et al., 2016). The meta-analysis by Marraccini and colleagues supported a positive and significant influence of prescription stimulant medication on processing speed accuracy, but this failed to translate to all aspects of cognitive performance or to performance overall. For other domains of cognition, the evidence is less clear. For example, while most studies demonstrate enhancement of executive function with stimulants in healthy participants, more than one-third demonstrate no effect on the cognitive processes of healthy nonelderly adults. Moreover, impairment of executive function has also been reported in healthy participants (Rogers et al., 1999), and participants with high performance at baseline or those homozygous for the met allele of the catechol-O-methyltransferase gene performed worse on drug compared with placebo (Mattay et al., 2000, 2003). Thus, while there is evidence that increasing catecholamine signaling can improve some aspects of cognitive function, depending on baseline performance, it remains uncertain in which healthy participants and patient populations these mechanisms might be used clinically to improve cognition.
Given the multi-day dosing design, there is potential for tolerance to D1R agonism. D1 effects. Compounds from this noncatechol chemical series have been shown to have reduced recruitment of β-arrestin signaling and correspondingly reduced receptor internalization and functional desensitization both in vitro and in vivo compared with other known D1R agonists (Gray et al., 2018). Tolerance to D1R effects was not observed in repeated dose preclinical studies with PF-06412562 using intermittent acute dosing (Pfizer, unpublished data), but there are no data available in these assays using a continuous dosing paradigm similar to that used in this study and the companion study in participants with schizophrenia. Additional confidence that tolerance may not be a confound in this study comes from recent clinical data on PF-06649751, a related noncatechol D1R partial agonist with a higher D1 intrinsic activity than PF-06412562, which improved Parkinson’s motor symptoms at 15 weeks of dosing, consistent with sustained functional D1R activation (Gray et al., 2018; in press), although Parkinson’s disease motor system effects may not be identical to effects in the circuitry underlying the cognition and reward/motivation endpoints included in this study. Another potential confound is the possibility of learning, training, or even receptor expression effects arising from the study design that precluded detection of significant changes in drug compared with placebo-treated groups. This study had a dense cognitive testing schedule, and the effect of sequential testing on these endpoints has not been well characterized. Longitudinal Hawthorne effects may also have contributed to the general pattern of improvement (e.g., due to relationship of the participants with study physicians or increased attention from research staff) (McCambridge et al., 2014). Indeed, placebo-treated groups demonstrated some improvement in several tasks compared with baseline as was expected for some tasks due to learning or training effects. We note that the CBS Battery and EVO were designed to improve aspects of cognitive function through repeated administration, and intensive cognitive training has been shown to impact D1R expression (Owen et al., 2010; Anguera et al., 2013). Studies to date, including the results we report here that evaluated improvement in brain training program endpoints vs traditional cognitive assessments, have not consistently demonstrated transfer of improvements to validated measures of cognitive performance (Owen et al., 2010; Soutschek et al., 2019). The effect of treatment with D1 agents depends on baseline dopamine levels to avoid side effects. This present pharmacological manipulation is systemic, so the D1 agonist can act both in prefrontal cortex, where it might impact decision-relevant cognitive processes, and in other regions containing D1Rs such as the striatum, where the D1 agonist might affect motivational rather than cognitive processes. Therefore, the effect profile will vary on whether the cognitive function is prefrontal or nigrostriatal direct pathway dependent. Furthermore, while participants were selected for low working memory capacity, the presence of practice effects may reflect the fact that, as healthy normal volunteers, participants may have compensatory strategies that preclude detection of functional improvements over baseline performance. In summary, we found that 3-mg and 15-mg PF-06412562 BID dosing were safe and well tolerated in healthy male participants with low working memory capacity. PF-06412562 did not improve cognition or motivation/reward domain function over a 5- to 7-day period in this population within this dose range. Interpretation of these results on cognition or motivation/reward domain function is complicated by the lack of consistency in meeting Stage 1 decision criteria across tasks intended to assess similar functions and by the greater improvement in performance on some measures of cognition observed in the placebo group relative to PF-06412562 dose groups. The interpretation is also complicated by the change from an acute and acute intermittent dosing paradigm (followed in the preclinical efficacy-informing experiments with the compound and all prior D1 agonist experiments) to a clinically conventional continuous exposure paradigm for which no preclinical efficacy assay data is available. This underscores the need to understand the impact of intermittent dosing on pharmacodynamic outcomes in follow-up clinical studies with this mechanism. The strengths of this study include the breadth and functional coherence of the endpoints, which enabled substantial confidence in determining the lack of beneficial effect observed with PF-06412562 in relation to processes underlying working memory and reward. While the neurofunctional domains approach described here is a comprehensive study design that could be applied to healthy volunteers or patients to interpret the effects of a treatment, we have reflected on the significant limitations of conclusions that can be drawn from the study we conducted. These include the lack of a positive control, the termination of the study after Stage 1 prior to the hypothesis-testing phase, the lack of consistency in meeting Stage 1 decision criteria across tasks that measure the same or similar constructs, and the inability to further assess potential false positives/negatives. Furthermore, even with multimodal assessments that covered many aspects of cognition and motivation/reward domains, no results were obtained that indicated effects of D1R agonism in the healthy human brain. Despite these limitations, we believe that the continued refinement and use of circuit and neurofunctional domains-based clinical trial designs will ultimately improve the translatability among preclinical models, healthy individuals, and patients. Building a deeper understanding of neural mechanisms modulated by target-relevant pharmacology is essential for the successful translation of preclinical discoveries into compelling proof-of-concept data that will establish new therapeutic concepts for neurologic and psychiatric diseases.
Acknowledgments
We thank all individuals who participated in this study. We thank collaborators around the globe, in particular Drs Anissa Abi-Dargham, Steven Arnold, Edward Awh, Deanna Barch, Roshan Cools, Randall Engle, Michael Frank, James Gold, Adam Hampshire, Scott Kellogg, Brian Knutson, Steven Luck, Adrian Owen, Diego Pizzagalli, Thomas Redick, Robert Rogers, Michael Treadway, and Edward Vogel, who contributed published and unpublished endpoints and protocols and provided conceptual and technical support during study design and execution. Medical writing support was provided by Sharmila Blows, PhD, of Engage and was funded by Pfizer.
This work was supported by Pfizer.
Statement of Interest
Z.X. is an employee of and holds stock in Pfizer. E.A., R.B.-G., G.H.B., C.C., N.D., D.L.G., B.H., G.D.H., R.K., W.L., S.D., and M.G.N. were Pfizer employees when this study was conducted. L.P. is an employee of CCTMG/PAREXEL and was contracted to Pfizer in connection with the development of the manuscript.
References
Author notes
Affiliation at the time this study was conducted