-
PDF
- Split View
-
Views
-
Cite
Cite
KIMIKO HIRAYAMA, KIYOSHI ISHIDA, NOBUHIRO TOMARU, Effects of pollen shortage and self-pollination on seed production of an endangered tree, Magnolia stellata, Annals of Botany, Volume 95, Issue 6, May 2005, Pages 1009–1015, https://doi.org/10.1093/aob/mci107
Close - Share Icon Share
Abstract
• Background and Aims Pollen limitation is a significant determinant of seed production, and can result from both insufficient pollen quantity (pollen shortage) and quality (mainly relating to self-pollination). For animal-pollinated tree species with large floral displays, pollen limitation may be determined by a balance between increased pollen quantity due to increased attractiveness for pollinators, countered by increased self-pollination due to increased geitonogamy. The contributions of pollen shortage and self-pollination on seed production were quantitatively examined in the natural pollination of an insect-pollinated, dichogamous, endangered tree, Magnolia stellata, which has a large, showy floral display.
• Methods Manual self- and cross-pollinations were conducted to determine the effects of selfing on seed production. The outcrossing rate was measured using microsatellite analyses of open-pollinated seeds, and the embryo mortality rate caused by self-pollination was indirectly estimated. The frequency of ovule mortality due to pollen shortage was also inferred using the embryo mortality and ovule survival rates from natural pollination.
• Key Results The average fruit set, seed set per fruit, and ovule survival rate per tree from hand cross-pollination were 1·37, 3·15, and 3·34 times higher than those from hand self-pollination, respectively, indicating that self-pollination causes inbreeding depression for fruit and seed set. The multilocus-outcrossing rate (tm) was intermediate, 0·632, and the primary selfing rate was 0·657. This indicates that frequent geitonogamous selfing occurs. The ovule mortality rate due to pollen shortage and the embryo mortality rate due to self-pollination were estimated to be 80·8 % and 45·9 %, respectively.
• Conclusions It is concluded that seed production of M. stellata is strongly limited by both pollen shortage and self-pollination. Inefficient beetle-pollination and the automimicry system via asynchronous flowering might be responsible for the high level of pollen shortage and frequent geitonogamy. This is despite a large, showy floral display and the dichogamous system of the species.
INTRODUCTION
The proportion of flowers and ovules that develop into fruits and seeds in flowering plants rarely reaches 1. Two proximate factors are suggested to explain this pattern: resource limitation and pollen limitation (Stephenson, 1981; Charlesworth, 1989; Campbell and Halama, 1993). From an evolutionary perspective, female reproductive success is primarily affected by resource limitation, not by pollen limitation, because pollen receipt is related to selection for floral traits of form and function in breeding systems (Bateman, 1948; Wilson, 1979). However, pollen limitation due to insufficient pollen delivery has been shown to be a common phenomenon in previous studies (Burd, 1994; Larson and Barrett, 2000). Burd (1994) demonstrated that 62 % of 258 species of angiosperms show evidence of pollen limitation, and stressed the importance of the stochastic pollinator environment as the cause of pollen limitation.
Animal-pollinated trees usually produce a large number of flowers because of their size, which should therefore lead to them receiving a large number of visits from pollinators. This could help relieve pollen limitation caused by insufficient quantity of pollen. However, more numerous floral displays generally result in a longer sequence of visits by pollinators to flowers within the same tree, which would result in increased geitonogamous self-pollination (de Jong et al., 1993; Klinkhamer and de Jong, 1993). If the species were to be self-incompatible, or self-compatible with the expression of high levels of early-acting inbreeding depression, seed production would be lowered by pollen limitation due to insufficient pollen quality induced by geitonogamy. Therefore, in tree species with large floral displays, the extent of pollen limitation would be determined by the balance between reduction in pollen shortage due to increasing attractiveness for pollinators, and increased self-pollination due to increasing geitonogamy. Understanding the reproductive ecology and evolution of reproductive traits of tree species requires quantitative evaluation of the absolute contribution of both pollen quantity and pollen quality for pollen limitation in seed production. However, most previous studies have evaluated pollen limitation only by increases in fruit and/or seed set using manual pollination experiments (that is, supplemental cross-pollination) compared with open-pollinated flowers (Zimmerman and Pyke, 1988; Burd, 1994). This only allows the evaluation of the combined effects of insufficient pollen quantity and pollen quality (see Ramsey and Vaughton, 2000). To clarify the full extent and causes of pollen limitation, it is necessary to separate the effects of pollen quality from those of quantity on the fruit and seed set of open-pollinated flowers.
Magnolia stellata is an endangered tree species (Environment Agency of Japan, 2000) endemic to the Tokai region of central Japan. Flowers are bisexual, protogynous and pollinated by insects. The overall floral display is showy because individual flowers are large and very numerous, and because flowering occurs before leaf flushing in early spring. Dichogamy might reduce intrafloral self-pollination and geitonogamy when a tree shows synchronous flowering (temporal separation of female- and male-phase in an individual tree; Rogstad, 1994). However, if flowering is asynchronous (female- and male-phase flowers coexist simultaneously on an individual tree; Kikuzawa and Mizui, 1990) and the behaviour of individual pollinators does not follow a regular pattern within a plant, dichogamy might have little effect on reducing geitonogamy (de Jong et al., 1993; Galloway et al., 2002). Therefore, for the dichogamous M. stellata, the extent of pollen limitation could be determined jointly by the effects of insufficient pollen quantity and quality, although it has not been clarified whether this species is self-incompatible or self-compatible. Clarification of its pollination biology with respect to pollen limitation, including self-compatibility, should provide an opportunity for a better understanding of factors that determine pollen limitation in dichogamous trees, and provide baseline information for conservation of this endangered species.
In this study, the extent of reduction in seed production due to insufficient pollen quantity and quality were examined in a naturally pollinated M. stellata population. First, manual self- and cross-pollinations experiments were conducted to determine the effects of selfing on seed production. Next, the outcrossing rate at the seed stage was measured and the embryo mortality rate due to self-pollination was estimated (insufficient pollen quality). Finally, the frequency of ovule mortality due to pollen shortage (insufficient pollen quantity) was inferred based on the method suggested by Ishida et al. (2003). This is the first study that has focused on quantifying the effects of both pollen shortage and self-pollination on seed production.
MATERIALS AND METHODS
Study species
Magnolia stellata Maxim. (Nooteboom, 1994; Magnolia tomentosa Thunb. is its synonym, Ueda, 1986) is a narrow endemic to the Tokai region of central Japan, where human impact is high, and is found in wetlands from 40–700 m in elevation (Japan Association for Shidekobushi Conservation, 1996). The species is a deciduous tree, grows up to 10 m in height, 20 cm in diameter at breast height, and often produces multiple stems. Flowering occurs in early spring, from March to April, and is immediately followed by leaf flushing. Flowers range from 10–15 cm in diameter, are hermaphroditic and protogynous, with 10–20 white to slightly pink tepals, 30–40 stamens, and 20–30 carpels per flower (Ishida et al., unpubl. data). Each carpel always has two ovules. An individual flower blooms for about 10 d. The tepals are initially closed in a fusiform shape up to approx. 2 d from anthesis, and then petals start to gradually open, becoming completely open approx. 4 d after anthesis. The surfaces of the stigma shrink and the anthers begin to dehisce viable pollen approx. 5 d after anthesis. The female and male flower phases last for 4–5 d and 5 d, respectively. Flowers are predominantly visited by small coleopterans belonging to the Staphylinidae (Ishida et al., unpubl. data). Fruits and seeds mature from late August to September. In this study, an aggregate of ripe carpels derived from the same flower are referred to as a fruit.
Study population
For the field study, a population was selected located along the Chikusui Pond (35°18′N, 137°03′E; elevation 140 m a.s.l.; about 0·075 km2 in size) in the northern part of Aichi Prefecture, central Japan. This population is now in a reserve, and contains 245 flowering individuals (in the case of aggregated multiple stems, we define them as one individual in this study) that are 8·45 ± 4·59 cm in diameter at breast height and possess 2·93 ± 2·80 stems (mean ± s.d.). Individuals of M. stellata are patchily distributed in wetlands at the periphery of the pond and are accompanied by Ilex spp., Quercus spp., Clethra barvinervis, Evodiopanax innovans and Rhododendron reticulatum. Based on data from almost all populations of M. stellata in the Tokai region, this population is regarded as a large one, although about 75 % of the other populations are small, with less than 100 individuals (Japan Association for Shidekobushi Conservation, 1996).
Pollination experiments
Hand self- and hand cross-pollination treatments were performed on 11 individual trees in the spring of 2003. Trees were randomly chosen in the population, the distance between them ranged from about 5–400 m, and represented different genets (genets were determined by four microsatellite loci; K. Hirayama and K. Ishida, unpubl. data). All trees were used for both seed and pollen, and each was pollinated using another tree as a pollen source for cross-pollination. Ten to 15 floral buds per seed tree were subjected to each pollination treatment. The buds were labelled and bagged with polypropylene netting. About 2–3 d after anthesis, when a flower was in the female stage and tepals were beginning to open, some tepals and all anthers were removed. All pistils were then pollinated with about 30 dehisced anthers from the appropriate pollen tree. The netting was removed when anthesis of the target flower was complete. Ten randomly selected flowers per tree were also labelled to examine fruit and seed following natural pollination. These flowers were kept intact.
Labelled fruits were harvested in late August. Seeds from the fruits were collected and viable seeds were distinguished by the colour of the aril (red, as opposed to dark brown for aborted seeds). Fresh weight of viable seeds was measured to the nearest 1 mg soon after removal from the arils. The number of carpels from the fruit was counted and the mean number per fruit was found to be 30·5 ± 5·9 (mean ± s.d.). The number of initial ovules (number of ovules at anthesis) from the fruits was estimated by multiplying the number of carpels by two, because each carpel always has two ovules. Percent fruit set [(number of fruits/number of flowers) × 100], and percent seed set per fruit [(number of viable seeds/number of initial ovules) × 100] were calculated. In addition, the percentage of ovules that survived per tree was calculated using the following equation: ovule survival rate per tree (%) = (fruit set) × (seed set per fruit) × 100.
Analyses of variance (ANOVAs) were performed to compare the four reproductive traits (fruit set, seed set per fruit, ovule survival rate per tree, and seed mass) among the different pollination treatments (hand cross-, hand self- and natural pollination). To meet the normality assumptions of ANOVA, fruit set, seed set per fruit, and ovule survival rate per tree were arcsine-transformed before performing the analyses (Sokal and Rohlf, 1995). Because the three pollination treatments were conducted on each tree and were therefore not independent of the tree, this factor can be regarded as a random block on the analyses. A generalized randomized block design (see Quinn and Keough, 2002) was used for the analyses that have replication within each combination of block and treatment (i.e. the analyses for seed set per fruit and seed mass), and an unreplicated random block design was used for the analyses without replication (i.e. fruit set and ovule survival rate per tree). When significant differences were observed, the Bonferroni method was used for multiple comparisons between pollination treatments with a Bonferroni correction of the alpha level (P = 0·05/3 = 0·017) for each variable.
Estimation of the magnitude of inbreeding depression
The magnitude of inbreeding depression caused by self-pollination at seed maturity was defined and calculated as δe = 1 − (ws/wo), where ws and wo are the ovule survival rate of selfed and crossed progeny, respectively. Standard errors were calculated using a jackknife resampling method for individual tree samples using the method of Manly (1997).
Estimation of outcrossing and primary selfing rate
Ten naturally pollinated fruits from each of 20 trees in the study population were harvested and available seeds were collected from each fruit in late August. Ten randomly selected seeds per tree were used for genetic analyses to determine the outcrossing rate at the seed stage.
Crude genomic DNA was extracted using a modification of the CTAB method (modified from Milligan, 1998). Because the embryos were too small for the procedure, both the embryos and the endosperms of the sampled seeds were ground in 650 μL of CTAB extraction buffer. The extracted DNA was purified using a silica matrix (FastDNA Kit, BIO 101, Inc., La Jolla, CA), following the manufacturer's protocol. Each DNA sample was individually subjected to polymerase chain reaction (PCR) amplification of two microsatellite loci (M10D6 and M6D8; Isagi et al., 1999) with fluorescently labelled primers. Amplified fragments were loaded on 5 % Long Ranger polyacrylamide gel, and electrophoresis was run for 2 h on an automated sequencer ABI 377 (Perkin-Elmer, Applied Biosystems, Inc., Foster City, CA). Microsatellite patterns were visualized with GeneScan™ 2.1.1 (Applied Biosystems).
The multilocus (tm) and single-locus (ts) outcrossing rates were then estimated, together with the extent of biparental inbreeding (tm − ts) using the MLTR computer program (Ritland, 2002) based on the procedure developed by Ritland and Jain (1981). Standard errors and 95 % confidence intervals (CI) were calculated by bootstrapping with 1000 repetitions for each genetic parameter. The primary selfing rates (s; self-fertilization rates) were also calculated by incorporating inbreeding depression for seed maturity (δe), as s = (1 − tm)/(1 − tmδe) (Maki, 1993).
Estimation of effects of self-pollination and pollen shortage in natural pollination
RESULTS
Differences in fruit and seed production with respect to hand cross- and self-pollination
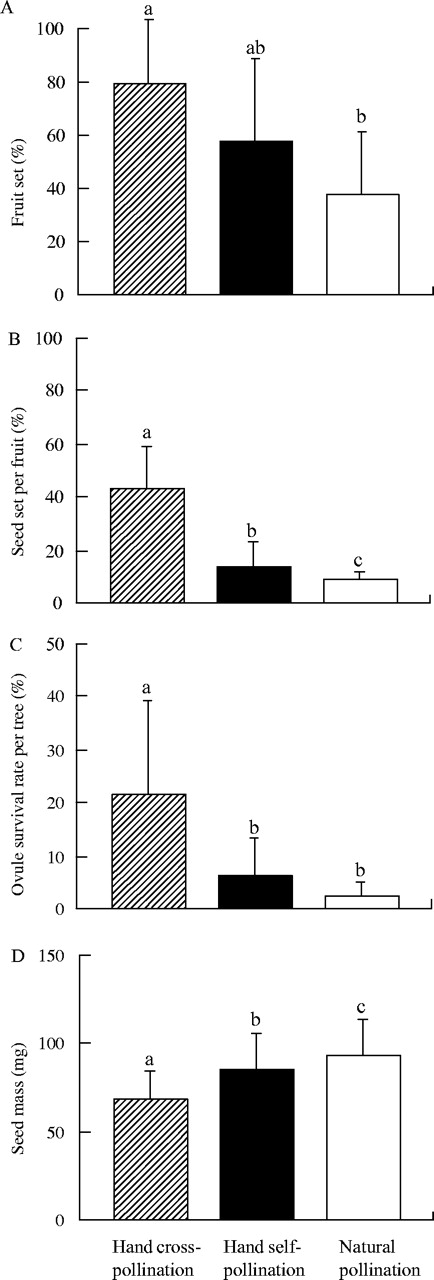
The average fruit set, seed set per fruit, and ovule survival rate per tree from hand cross-pollination were 79·5 %, 43·3 % and 21·7 %, respectively, and those from hand self-pollination were 57·8 %, 13·7 % and 6·5 %, respectively (Fig. 1). There was no significant difference for the fruit set between the types of hand pollination The seed set and the ovule survival rate from hand self-pollination were significantly smaller than those from hand cross-pollination (Table 1 and Fig. 1). The average hand crossed seed mass was 68·1 mg, which was 15 mg lighter than that of the hand selfed seed mass. This difference was statistically significant.
Changes in mean values of (A) fruit set, (B) seed set per fruit, (C) ovule survival rate per tree, and (D) seed mass for 11 sampled trees with respect to the three types of pollination. Vertical bars show the standard deviations. Different letters above the columns indicate significant differences between the types of pollination for each variable (P < 0·017, Bonferroni test).
Analyses of variance for four reproductive traits (fruit set, seed set per fruit, ovule survival rate per tree and seed mass)
| Variable and source . | d.f. . | MS . | F . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| Fruit set | ||||||||
| Pollination type | 2 | 1·010 | 13·486 | <0·001 | ||||
| Tree | 10 | 0·251 | 3·347 | 0·010 | ||||
| Error | 20 | 7·492 × 10−2 | ||||||
| Seed set per fruit | ||||||||
| Pollination type | 2 | 2·997 | 179·019 | <0·001 | ||||
| Tree | 10 | 0·104 | 6·231 | <0·001 | ||||
| Pollination type × Tree | 18 | 8·418 × 10−2 | 5·029 | <0·001 | ||||
| Error | 179 | 1·674 × 10−2 | ||||||
| Ovule survival rate per tree | ||||||||
| Pollination type | 2 | 0·291 | 21·636 | <0·001 | ||||
| Tree | 10 | 4·805 × 10−2 | 3·571 | 0·007 | ||||
| Error | 20 | 1·345 × 10−2 | ||||||
| Seed mass | ||||||||
| Pollination type | 2 | 8508·373 | 60·921 | <0·001 | ||||
| Tree | 10 | 3997·922 | 28·625 | <0·001 | ||||
| Pollination type × Tree | 18 | 498·343 | 3·568 | <0·001 | ||||
| Error | 168 | 139·663 | ||||||
| Variable and source . | d.f. . | MS . | F . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| Fruit set | ||||||||
| Pollination type | 2 | 1·010 | 13·486 | <0·001 | ||||
| Tree | 10 | 0·251 | 3·347 | 0·010 | ||||
| Error | 20 | 7·492 × 10−2 | ||||||
| Seed set per fruit | ||||||||
| Pollination type | 2 | 2·997 | 179·019 | <0·001 | ||||
| Tree | 10 | 0·104 | 6·231 | <0·001 | ||||
| Pollination type × Tree | 18 | 8·418 × 10−2 | 5·029 | <0·001 | ||||
| Error | 179 | 1·674 × 10−2 | ||||||
| Ovule survival rate per tree | ||||||||
| Pollination type | 2 | 0·291 | 21·636 | <0·001 | ||||
| Tree | 10 | 4·805 × 10−2 | 3·571 | 0·007 | ||||
| Error | 20 | 1·345 × 10−2 | ||||||
| Seed mass | ||||||||
| Pollination type | 2 | 8508·373 | 60·921 | <0·001 | ||||
| Tree | 10 | 3997·922 | 28·625 | <0·001 | ||||
| Pollination type × Tree | 18 | 498·343 | 3·568 | <0·001 | ||||
| Error | 168 | 139·663 | ||||||
Arcsine-transformed data were analysed for fruit set, seed set per fruit and ovule survival rate per tree.
Analyses of variance for four reproductive traits (fruit set, seed set per fruit, ovule survival rate per tree and seed mass)
| Variable and source . | d.f. . | MS . | F . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| Fruit set | ||||||||
| Pollination type | 2 | 1·010 | 13·486 | <0·001 | ||||
| Tree | 10 | 0·251 | 3·347 | 0·010 | ||||
| Error | 20 | 7·492 × 10−2 | ||||||
| Seed set per fruit | ||||||||
| Pollination type | 2 | 2·997 | 179·019 | <0·001 | ||||
| Tree | 10 | 0·104 | 6·231 | <0·001 | ||||
| Pollination type × Tree | 18 | 8·418 × 10−2 | 5·029 | <0·001 | ||||
| Error | 179 | 1·674 × 10−2 | ||||||
| Ovule survival rate per tree | ||||||||
| Pollination type | 2 | 0·291 | 21·636 | <0·001 | ||||
| Tree | 10 | 4·805 × 10−2 | 3·571 | 0·007 | ||||
| Error | 20 | 1·345 × 10−2 | ||||||
| Seed mass | ||||||||
| Pollination type | 2 | 8508·373 | 60·921 | <0·001 | ||||
| Tree | 10 | 3997·922 | 28·625 | <0·001 | ||||
| Pollination type × Tree | 18 | 498·343 | 3·568 | <0·001 | ||||
| Error | 168 | 139·663 | ||||||
| Variable and source . | d.f. . | MS . | F . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| Fruit set | ||||||||
| Pollination type | 2 | 1·010 | 13·486 | <0·001 | ||||
| Tree | 10 | 0·251 | 3·347 | 0·010 | ||||
| Error | 20 | 7·492 × 10−2 | ||||||
| Seed set per fruit | ||||||||
| Pollination type | 2 | 2·997 | 179·019 | <0·001 | ||||
| Tree | 10 | 0·104 | 6·231 | <0·001 | ||||
| Pollination type × Tree | 18 | 8·418 × 10−2 | 5·029 | <0·001 | ||||
| Error | 179 | 1·674 × 10−2 | ||||||
| Ovule survival rate per tree | ||||||||
| Pollination type | 2 | 0·291 | 21·636 | <0·001 | ||||
| Tree | 10 | 4·805 × 10−2 | 3·571 | 0·007 | ||||
| Error | 20 | 1·345 × 10−2 | ||||||
| Seed mass | ||||||||
| Pollination type | 2 | 8508·373 | 60·921 | <0·001 | ||||
| Tree | 10 | 3997·922 | 28·625 | <0·001 | ||||
| Pollination type × Tree | 18 | 498·343 | 3·568 | <0·001 | ||||
| Error | 168 | 139·663 | ||||||
Arcsine-transformed data were analysed for fruit set, seed set per fruit and ovule survival rate per tree.
Based on the average value of individual tree samples, the magnitude of inbreeding depression caused by self-pollination at seed maturity (δe) was 0·700 ± 0·085 (mean ± s.e.). The actual range and the standard deviation of δe (values obtained from single trees) for 11 sampled trees were −0·413 to 0·951 and 0·390, respectively, indicating large differences in the magnitude of inbreeding depression at this stage among trees in the population.
Fruit and seed production from natural pollination
The average fruit set, seed set per fruit and ovule survival rate per tree from natural pollination were 38·2 %, 8·7 % and 2·6 %, respectively (Fig. 1). The seed set was significantly lower than that from hand self-pollination, which was 3·15 times lower than from hand cross-pollination (Table 1 and Fig. 1). The fruit set and the ovule survival rate from natural pollination were also smaller than those from hand self-pollination, although the differences were not statistically significant. The seeds from natural pollination were significantly heavier than those from both hand cross- and hand self-pollination.
The multilocus outcrossing rate (tm) was 0·632 ± 0·072 (mean ± s.e.). The value of tm was significantly different from unity as the 95 % confidence interval was 0·527–0·761. The extent of biparental inbreeding (tm – ts) was 0·039 ± 0·017 (mean ± s.e.), which was low, but significant because the 95 % confidence interval was 0·006–0·063. Using the values for tm and δe, we obtained a primary selfing rate (s) of 0·657 ± 0·065 (mean ± s.e.).
Assuming that M. stellata was completely self-compatible, the ovule mortality rate due to pollen shortage (u) was estimated as 0·808 ± 0·078 (mean ± s.e.), with a confidence interval of 0·633–0·983. For the stage up to seed maturation, it was estimated that 45·9 ± 10·2 % (g; mean ± s.e.) embryos died due to self-pollination. The 95 % confidence interval of the g value was large, ranging from 23·1–68·7 %.
DISCUSSION
Effects of self-pollination on seed production
The seed set and the ovule survival rate from hand self-pollination was significantly lower than from hand cross-pollination, while seeds from hand self-pollination weighed more. The difference in seed mass between the pollination types was small compared with the difference in the other two traits. These results indicate that self-pollination causes a reduction in fitness up to seed maturation for M. stellata.
The decrease in fitness in seed production from self-pollination could be explained by self-incompatibility and/or inbreeding depression due to the expression of recessive deleterious alleles following increased homozygosity. Self-incompatibility usually results from maternal tissue/pollen interactions and typically occurs at the surface of the stigma or in the style (de Nettancourt, 1977). However, in a separate study, we observed that the amount and elongation rate of self-pollinated pollen tubes in the styles did not differ from those that were cross-pollinated. Using UV microscopy (Dafni, 1992), both self and cross pollen tubes were found to reach the base of the style (K. Hirayama and K. Ishida, unpubl. data). Thus, it is unlikely that a common self-incompatibility system operates in M. stellata. On the other hand, recent studies have pointed out the existence of late-acting self-incompatibility, in which self-pollen rejection occurs after the pollen tubes enter the ovary (Seavey and Bawa, 1986; Barrett, 1998; Sage et al., 1999). However, all individual trees of M. stellata produced fruits and seeds, and the fruit set and seed set per tree were 57·8 % and 13·7 %, respectively: these results do not indicate a late-acting self-incompatibility system in which the seed set from self-pollination is zero (or near zero) in most individuals (Seavey and Bawa, 1986). In addition, our observation of the wide range and large standard deviation of δe for individual trees at seed maturity rather supports evidence of inbreeding depression due to the expression of recessive deleterious alleles. This is consistent with other studies that have shown that the number of lethal equivalents varies between individuals (Lynch and Walsh, 1998). Thus the decrease in seed production due to self-pollination for M. stellata is probably explained by inbreeding depression rather than late-acting self-incompatibility.
Theoretical studies have suggested that predominant outcrossing will evolve when the δ value is larger than 0·5, with respect to the balance between the cost (inbreeding depression) and benefit (gene transmission advantage to the next generation) of selfing (Lande and Schemske, 1985). Our results demonstrated that the average value of δe for M. stellata was 0·700, which was larger than 0·5 and similar to the δ value at the embryo stage in predominantly outcrossing gymnosperms (mean δ = 0·58, n = 14; Husband and Schemske, 1996), although the magnitude of inbreeding depression would be overestimated if M. stellata trees are not completely self-compatible. Despite this severe inbreeding depression, our genetic analyses demonstrated that M. stellata trees exhibit a high primary selfing rate (65·7 %). This high selfing rate cannot be explained by the theoretical prediction that a δ value larger than 0·5 leads to predominant outcrossing, but rather by the context of the pollination process (Holsinger, 1992; Lloyd, 1992; Harder and Barrett, 1996): pollination mechanisms for promoting outcrossing would result in geitonogamous selfing.
For temperate Magnolia species, whose female stage flowers offer neither pollen nor nectar, automimicry (non-rewarding female-stage flowers mimic the rewarding male-stage flowers) that is based on asynchronous flowering is required to attract pollinators (Bernhardt and Thien, 1987; Kikuzawa and Mizui, 1990). However, Ishida et al. (2003) pointed out that the automimicry system of temperate Magnolia would cause geitonogamy via asynchronous flowering, which would result in fitness reduction due to inbreeding depression. They also proposed the idea that the cost (inbreeding load) of the automimicry system is compensated for by the saving of pollinator rewards at the female phase of the flowers. As flowers of M. stellata open asynchronously within an individual tree (K. Hirayama and K. Ishida, unpubl. data) and produce no nectar as do other temperate magnolias, geitonogamous selfing would also be a by-product of an automimicry system for interfloral pollination. The protogyny of this tree species, which has a large floral display with asynchronous flowering, might have little effect on reducing geitonogamy, as Galloway et al. (2002) found in the herbaceous plant Campanula americana, which has a loosely structured inflorescence. Therefore, we suggest that substantial limitation to seed production occurs by insufficient pollen quality because geitonogamous self-pollination is unavoidable in M. stellata, even if the trees are pollinated sufficiently with respect to pollen quantity.
Effects of pollen shortage on seed production
Magnolia stellata flowers in the studied population were visited by a substantial number of small beetles belonging to the Staphylinidae, and a few flies and bees (K. Ishida et al., unpubl. data). We observed these small beetles foraging for pollen and/or crawling on stigmas and stamens in both male and female flowers, indicating that they are probably the main pollinators of M. stellata. In other Magnolia species predominantly pollinated by beetles, pollen shortage or inefficient pollination has been reported. For example, a substantial pollen shortage was responsible for a low fruit set in Magnolia praecocissima var. borealis (Ishida, 1996), and only a few pollen grains were deposited on 44 % of stigmas in Magnolia tamaulipana (Dieringer et al., 1999). Although Thien (1974) predicted that flowers of Magnolia are highly specialized for pollination by beetles, they might not be very effective pollinators. In other plant taxa, pollination by beetles has been observed to be less efficient than by bees (Ramsey, 1988; Proctor et al., 1996). In addition, as M. stellata trees flower in early spring, pollinator service may be unreliable because of unpredictable climatic conditions, as pointed out by Motten (1986) and McCall and Primack (1992) in other plants that flower at this time. Moreover, a review by Larson and Barrett (2000) suggested that tree species with large floral displays are likely to suffer pollen limitation compared with herbaceous species because visitation rates of pollinators per flower do not increase in proportion to increases in display size. These pollination characteristics of M. stellata help to explain the high level of pollen shortage despite a large floral display that might facilitate the attraction of pollinators.
CONCLUSIONS
This study indicates that seed production in M. stellata, a tree with a large floral display, is limited by both pollen shortage and self-pollination. First, 80·8 % of ovules failed to develop into seeds because of pollen shortage, although a large floral display is generally expected to reduce an insufficiency of pollen quantity. Second, the large floral display with an automimicry system caused frequent geitonogamous self-pollination, which resulted in a decrease in seed production to 45·9 %. The total of these mortality rates of ovules was 89·6 %, which is the extent of pollen limitation for seed production in M. stellata. This value for pollen limitation would partly depend on environmental factors such as climatic conditions, and might fluctuate from year to year. However, such a high level of pollen limitation might occur regularly in M. stellata because pollen limitation mainly results from its own pollination characteristics. In the context of conservation biology, further fragmentation and/or isolation of M. stellata populations might seriously decrease their seed production. Fragmentation and/or isolation of populations often reduce pollinator activity (Sih and Baltus, 1987; Kunin, 1993; Rathcke and Jules, 1993). Quantitative evaluation of changes in the levels of pollen shortage and self-pollination with decreasing population size and density of individual trees is urgently required in order to formulate proposals for the conservation of this endangered species.
We are grateful to Ayako Kanazashi for helpful comments, Suzuki Setsuko and Naoki Ohnishi for their advice on the genetic analysis, Yoshiko Shimada for assistance with the laboratory work, Yoshihiro Takahata for advice in the using UV microscopy, Koji Yamamura, Kazuki Miyamoto and Hiroki Ito for advice in statistical analyses. Our thanks are given to the Aichi Prefectural Forest Office and Kasugai Nature Center for Youth for permitting this study. We also indebted to professor D.A. Levin, Dr G. Dieringer, and two anonymous referees for making helpful suggestions on the manuscript. This study was supported by a grant from the Ministry of the Environment of Japan (representative, Ayako Kanazashi) and by a grant from the Japan Society for Promotion of Science Research Fellowships for Young Scientists to Kimiko Hirayama.
LITERATURE CITED
Barrett SCH.
Bernhardt P, Thien LB.
Burd M.
Campbell DR, Halama KJ.
Charlesworth D.
Dieringer G, Cabrera RL, Lara M, Loya L, Reyes-Castillo P.
Environment Agency of Japan.
Galloway LF, Cirigliano T, Gremski K.
Harder LD, Barrett SCH.
Holsinger KE.
Husband BC, Schemske DW.
Isagi Y, Kanazashi T, Suzuki W, Tanaka H, Abe T.
Ishida K.
Ishida K, Yoshimaru H, Itô H.
Japan Association for Shidekobushi Conservation.
de Jong TJ, Waser NM, Klinkhamer PGL.
Kikuzawa K, Mizui N.
Klinkhamer PGL, de Jong TJ.
Kunin WE.
Lande R, Schemske DW.
Larson BMH, Barrett SCH.
Lloyd DG.
Lynch M, Walsh B.
McCall C, Primack RB.
Maki M.
Manly BFJ.
Milligan BG.
Motten AF.
Nooteboom HP.
Quinn GP, Keough MJ.
Ramsey MW.
Ramsey M, Vaughton G.
Rathcke BJ, Jules ES.
Ritland K.
Ritland K, Jain S.
Rogstad SH.
Sage TL, Strumas F, Cole WW, Barrett SCH.
Seavey SR, Bawa KS.
Sih A, Baltus M-S.
Stephenson AG.
Ueda K.
Author notes
1Kansai Research Center, Forestry and Forest Products Research Institute, Momoyama, Fushimi, Kyoto 612-0855, Japan and 2Laboratory of Forest Ecology and Physiology, Graduate School of Bioagricultural Science, Nagoya University, Nagoya 464-8601, Japan