Abstract
Introduction
Streptococcus pyogenes (group A Streptococcus, GAS) is responsible for a variety of highly communicable infections, accounting for 5–15 and 20–30% of sore throat hospital visits in adults and children, respectively. Prompt diagnosis of GAS can improve the quality of patient care and minimize the unnecessary use of antibiotics.
Objective
Our objective was to develop an alternative nucleic acid amplification method for the diagnosis of GAS.
Method
We developed and evaluated a strand invasion based amplification (SIBA) assay to rapidly and specifically detect GAS. The performance of the developed GAS SIBA assay was compared with an established GAS polymerase chain reaction (PCR) assay.
Results
The GAS SIBA assay detected small amounts (ten copies) of S. pyogenes DNA within 13 min. The rapid detection time was achieved in part by optimization of magnesium concentration and reaction temperature. The sensitivity and specificity of the GAS SIBA assay for detection of S. pyogenes from clinical specimens were both 100%, and clinical specimens were detected within ~ 8 min of starting the reaction.
Conclusion
Because the GAS SIBA assay is performed at low and constant temperature, it can be used both in centralized laboratories and for point-of-care testing. Furthermore, given its short detection time and strong analytical performance, the GAS SIBA assay could help to improve patient care and minimize unnecessary prescription of antibiotics.
Similar content being viewed by others
A novel isothermal nucleic acid amplification method, strand invasion based amplification (SIBA), was developed for the rapid and specific detection of Streptococcus pyogenes. |
The method specifically detected S. pyogenes within 8 min from clinical specimens and can be potentially use within a point-of-care setting. |
1 Introduction
Streptococcus pyogenes, also known as group A Streptococcus (GAS), is responsible for a variety of highly communicable infections, including acute pharyngitis (sore throat) [1, 2]. GAS infection is a major healthcare burden, accounting for 5–15 and 20–30% of sore throat hospital visits in adults and children, respectively [3]. Untreated GAS infection can lead to life-threatening diseases such as acute rheumatic fever and acute post-streptococcal glomerulonephritis [4, 5]. Because S. pyogenes has remained fairly susceptible to broad-spectrum antibiotics, patients with GAS infections are often treated with antibacterial agents such as penicillin or amoxicillin [3]. However, the clinical signs and symptoms of streptococcal pharyngitis are similar to those of non-streptococcal pharyngitis, particularly viral pharyngitis [6]. Consequently, clinicians rely on laboratory tests to confirm or exclude GAS, make appropriate treatment decisions, and avoid unnecessary use of antibiotics.
Common laboratory methods for identification of GAS pharyngitis include culture of bacteria or rapid antigen detection tests (RADTs) using material obtained from throat swabs. Throat culture remains the most prominent method, but has a relatively long turnaround time (TAT) of 1–2 days. RADTs offer significantly shorter TAT and can be performed in low-resource or point-of-care settings. However, in comparison with the throat culture method, RADTs have inadequate sensitivity, between 70 and 90% [7,8,9]. According to the American Academy of Pediatrics (AAP) recommendations, children with negative GAS RADT results should be retested for S. pyogenes by throat culture [5]. Due to their superior sensitivity in comparison with RADTs, nucleic acid amplification tests (NAATs), e.g., real-time polymerase chain reaction (PCR), have recently been introduced for identification of GAS infections [10]. However, the TATs of NAATs are still significantly longer than those of RADTs, and are limited to specialized laboratories because of the requirement for sophisticated instrumentation. NAATs with a rapid TAT could aid in timely diagnosis of S. pyogenes, thereby improving patient care and treatment decisions.
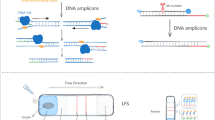
In this study, we developed and evaluated a rapid NAAT for the detection of S. pyogenes. Our NAAT is based on a novel isothermal nucleic acid amplification method called strand invasion based amplification (SIBA) [11, 12]. SIBA utilizes a recombinase-coated, single-stranded invasion oligonucleotide (IO) to separate the target duplex. Gp32 and UvsX are key proteins required for performing SIBA reactions. Gp32 abolish the formation of secondary structures within the IO, enabling UvsX to cooperatively bind the IO. The UvsX-IO filament complex catalyzes the strand separation of the homologous target duplex enabling target-specific primers to bind and extend the target via DNA polymerase. These events subsequently lead to an isothermal exponential amplification of the target nucleic acid (Fig. 1). The method was capable of detecting S. pyogenes within minutes and exhibited high analytical sensitivity and specificity.
DNA amplification by strand invasion based amplification method. 1. The SIBA reaction requires two target-specific primers and IO. Gp32 binds to all single-stranded DNA. 2. The recombinase protein, UvsX, coats the IO, displacing the bound Gp32. The primers are too short to act as substrates for UvsX. 3. The recombinase-coated IO invades the complementary region of the target duplex. The invasion process facilitates the separation of the target duplex, enabling target-specific primers to bind the target. 4. The strand displacement polymerase extends the dissociated target duplex from the primers. 5. This event leads to the production of two copies of the target duplex. Recombinase-mediated target duplex separation and polymerase-mediated extension are the basis for exponential amplification. Image and description were modified from Eboigbodin et al. [13]. IO invasion oligonucleotide, SIBA strand invasion based amplification
2 Materials and Methods
2.1 Microbial Strains and Clinical Specimens
A total of 27 microbial strains were used to develop the GAS SIBA assay. S. pyogenes ATCC 19615 and S. pyogenes NCTC 9994 were used as positive controls and to establish the analytical sensitivity of the assay. A total of 25 non-S. pyogenes strains, including those responsible for viral pharyngitis, were used to determine the analytical specificity of the assay. A total of 45 retrospective throat swab specimens obtained from the Discovery Life Sciences Biobank (Discovery Life Sciences, USA), previously determined by throat culture to be positive or negative for S. pyogenes, were used to evaluate clinical performance of both the GAS SIBA and GAS PCR assays. All specimens were used in accordance with Discovery Life Sciences Biobank Bioethics Policy.
2.2 DNA Extraction
DNA was extracted from microbial strains using the QIAamp DNA Mini Kit (Qiagen, Germany). Viral RNA was extracted from viral strains using the QIAamp Viral RNA Mini Kit (Qiagen). DNA was also extracted from throat swabs using the QIAamp DNA Mini Kit. Prior to extraction, dry throat swabs were first re-suspended in 500 µl of buffer (0.5% Triton × 100, 0.5% Tween-20), and 200 µl of the suspension was used for nucleic acid extraction.
2.3 Strand Invasion Based Amplification (SIBA) Assay Design
The GAS SIBA assay was designed to specifically amplify and detect the S. pyogenes pyrogenic exotoxin B (speB) gene. Forward (GGAGGATTTGTTATCGT) and reverse primer (AATGATCCGCTGGTAGAGT) pairs and the IO (CCCCCCCCCC TTTCAGGAGATAAACGTTCTCCAGAAATTCmUmAmGmGmAmUmAmCmUmCmUmAmC) were designed according to a previously published protocol [12]. The IO includes a 10-mer polynucleotide non-complementary to the target region (poly-C), which facilitates optimal coating of the IO by the recombinase [12,13,14]. In addition, the 3′-end of the IO (last 13 nucleotides) contains 2′-O-methyl RNA nucleotides that prevent the IO from acting as a primer or being extended by DNA polymerase (m, denotes 2′-O-methyl RNA nucleotides).
2.4 SIBA Reaction
The GAS SIBA assay was performed using the SIBA reagent kit (Orion Diagnostica Oy, Finland). The forward primer, reverse primer, and IO were each present at 300 nM. The T4 Gp32 (Roche CustomBiotech, Germany) and UvsX (Orion Diagnostica Oy, Finland) proteins were both present at concentrations of 300 ng/ml. Bacillus subtilis DNA Polymerase I, Large Fragment (BSU) is the DNA polymerase used in SIBA. Two microliters of each DNA/RNA extract (corresponding from 1 to 105 copies per reaction) was assayed in a total reaction volume of 20 μl. SIBA reaction products were detected using SYBR Green (1:100,000 dilution). Reactions were incubated at temperatures between 37 and 50 °C for 30 min, and fluorescence measurements were collected every minute on a Bio-Rad CFX96 system (Bio-Rad Laboratories, UK). Except otherwise stated, magnesium acetate was present at 12.5 mM.
2.5 Polymerase Chain Reaction (PCR)
According to Centers for Disease Control and Prevention recommendations [15], the performance of GAS SIBA assay was compared with that of a previously published GAS PCR assay. Briefly, PCR was conducted in a total reaction volume of 20 µl containing 10 µl of 2 × iTaq universal probe qPCR Supermix (Bio-Rad Laboratories, Finland). Primers (forward-GCACTCGCTACTATTTCTTACCTCAA; reverse-GTCACAATGTCTTGGAAACCAGTAAT) and probe (FAM-CCGCAAC-BHQDT-CATCAAGGATTTCTGTTACCA-SPC3I) were present at 400 and 200 nM, respectively. Reaction conditions were as follows: 45 °C for 10 min, 94 °C for 10 min, and 45 cycles of denaturation at 95 °C for 30 s and amplification at 60 °C for 60 s. Two microliters of each DNA/RNA from the SIBA extract used in the SIBA assay was assayed in a total reaction volume of 20 μl. PCR reactions were performed using the Bio-Rad CFX96 system (Bio-Rad Laboratories).
3 Results
3.1 Optimization of the Group A Streptococcus (GAS) SIBA Assay
The GAS SIBA assay was optimized for rapid and specific detection of S. pyogenes by determining the optimal reaction temperature and magnesium acetate concentration (Fig. 2). Reactions were incubated at temperatures between 37 and 50 °C for 30 min in the presence or absence of 200 copies of S. pyogenes DNA. Each reaction temperature condition was performed in quadruplicate. The assay detected S. pyogenes DNA in reactions performed between 37 and 48 °C. Above 48 °C, no S. pyogenes DNA was detected, presumably due to enzyme degradation. The fastest detection of S. pyogenes DNA occurred in reactions incubated between 42 and 45 °C.
We also examined the impact of magnesium acetate on amplification efficiency in the GAS SIBA assay. In these reactions, we used magnesium acetate at concentrations between 2.5 and 30 mM in the presence or absence of 200 copies of S. pyogenes DNA. The assay detected S. pyogenes DNA at magnesium acetate concentrations above 5 mM, with the fastest detection occurring between 10 and 15 mM. Magnesium acetate became inhibitory at concentrations above 20 mM. The impact of magnesium acetate on the SIBA reaction is also likely to be dependent on the activity of the DNA polymerase, BSU. Based on these findings, subsequent experiments were performed at 43 °C with 12.5 mM magnesium acetate.
3.2 Analytical Sensitivity and Specificity
We determined the analytical sensitivity of the GAS SIBA assay using quantified DNA extracted from S. pyogenes ATCC 19615 and S. pyogenes NCTC 9994. Specifically, the sensitivity of the assay was determined in three independent experiments by adding serial dilutions of DNA (from 1 to 105 copies per reaction). Each dilution was performed in quadruplicate, and the results are shown in Fig. 3. The GAS SIBA assay detected as few as ten copies of S. pyogenes ATCC 19615 or S. pyogenes NCTC 9994 DNA within 13 and 12 min, respectively. Higher copy numbers of S. pyogenes DNA were detected in less than 10 min. These results demonstrate that the GAS SIBA assay can detect S. pyogenes with a TAT similar to those of RADTs and faster than those of most NAATs for detection of S. pyogenes.
Sensitivity of SIBA and PCR for the detection of Streptococcus pyogenes. a SIBA detection of S. pyogenes ATCC 19615 DNA. b SIBA detection of S. pyogenes NCTC 9994 DNA. c PCR detection of S. pyogenes ATCC 19615 DNA. d PCR detection of S. pyogenes NCTC 9994 DNA. Ct cycle threshold, NTC no template control, PCR polymerase chain reaction, SIBA strand invasion based amplification
Next, we investigated analytical specificity by challenging the GAS SIBA assay with DNA or RNA extracted from 25 microbial strains (Table 1). The microbial panel included seven Streptococcus strains other than S. pyogenes, as well as other bacteria and viruses known to cause pharyngitis. None of these 25 microbes were detected by the GAS SIBA assay, suggesting that the assay is highly specific for S. pyogenes.
3.3 GAS SIBA Clinical Specimen Study
We then evaluated the GAS SIBA assay using 45 retrospective throat swab specimens previously determined by throat culture to be positive or negative for S. pyogenes. The specimens were retested with the GAS SIBA and reference real-time PCR assay (Table 2). Both assays yielded identical results in terms of the number of positive and negative specimens detected. The sensitivity and specificity of the GAS SIBA assay relative to the reference GAS PCR assay were 100% (95% confidence interval [CI] 85.7–100.0) and 100% (95% CI 85.1–100.0), respectively, indicating that the GAS SIBA assay is highly specific and sensitive, with performance similar to that of NAAT reference methods. The GAS SIBA assay had a significantly faster detection time than the PCR reference method (Fig. 4): the average time to positive results for the GAS SIBA was 8 min, whereas the reference PCR assay took around 90 min to obtain a positive result (cycle threshold [Ct] = ~ 27).
Rapid detection of GAS. a Distribution of time required to detect GAS-positive throat specimens by SIBA and PCR assays. b Comparison of detection times of the GAS SIBA and GAS PCR assays. *GAS SIBA and GAS PCR assay detection times are reported in minutes and Ct, respectively. Ct cycle threshold, GAS group A Streptococcus, PCR polymerase chain reaction, SIBA strand invasion based amplification
4 Discussion
S. pyogenes, also called group A Streptococcus (GAS), causes a wide range of infections in humans. Consequently, timely diagnosis of GAS infection plays an important role in improving patient care and limiting the inappropriate use of antibiotics. Due to their short TAT and ease of use, RADTs have been used to facilitate rapid detection of S. pyogenes. However, due to their inadequate sensitivity, negative results obtained by RADT still require further confirmation by culture [5]. NAATs offer superior sensitivity over RADTs but have longer TATs. Furthermore, due to their requirement for skilled personnel and large instruments, the use of NAATs is often confined to large central laboratories. Rapid NAATs with high analytical performance could facilitate prompt diagnosis of GAS infection, improving patient care and clinical decision-making.
In this study, we developed and evaluated an isothermal NAAT based on SIBA for rapid detection of S. pyogenes. This method uses a recombinase-coated oligonucleotide to catalyze dissociation of a specific target sequence within the S. pyogenes genome, enabling target-specific primers to exponentially amplify the target sequence. This method was previously shown to be rapid and highly sensitive for the detection of infectious diseases [11,12,13, 16, 17].
The GAS SIBA assay exhibited high analytical sensitivity and specificity for detection of S. pyogenes, and did not detect non–S. pyogenes strains. Moreover, it could detect as few as ten copies of S. pyogenes DNA within 13 min of starting the reaction, significantly faster than previously reported NAATs. This short TAT was achieved in part by optimization of the magnesium concentration and reaction temperature. The GAS SIBA assay was functional at a wide range of magnesium concentrations (5–30 mM). Magnesium ion is an important cofactor for the recombinase and DNA polymerase used in SIBA, and is also an important cofactor for the DNA polymerase used in PCR. The presence of trace amounts of ethylenediaminetetraacetic acid or other ions (e.g., calcium) originating from specimens or extraction inhibits PCR reactions [18], either by chelating or competing with magnesium ions required by the DNA polymerase. In PCR, the optimal concentration of magnesium is usually between 1 and 3 mM, whereas higher concentrations can be inhibitory [19, 20]. Therefore, PCR reactions can easily be inhibited by specimens containing small amounts of chelating agents or competing ions. By contrast, because SIBA is tolerant to high levels of magnesium, residual amounts of chelators or competing ions would have minimal effects on the reaction. Thus, SIBA reactions may be more tolerant to inhibitors than the PCR method. Furthermore, because the GAS SIBA assay is functional over a wide range of temperatures (37–48 °C), highly accurate temperature-controlled instruments are not necessary for SIBA reactions.
The GAS SIBA assay was further evaluated using 45 retrospective throat swab specimens previously determined to be positive or negative for S. pyogenes. These specimens were retested with the GAS SIBA and a reference GAS PCR assay. The sensitivity and specificity of the GAS SIBA assay using these specimens were both 100%, indicating that the analytical performance of SIBA is comparable to those of reference NAAT methods. To confirm this, however, the GAS SIBA assay needs to be further validated with larger numbers of clinical specimens.
The GAS SIBA assay detected positive clinical specimens in a mean time of 8 min, significantly faster than the PCR assay, which took more than 90 min. However, DNA from the clinical specimens were first extracted using a commercial DNA isolation kit prior to SIBA amplification, further increasing the total detection time. A rapid specimen processing protocol will need to be use in order to maximize the fast amplification nature of SIBA. The short detection time and strong analytical performance of the GAS SIBA could facilitate timely diagnosis, improve patient care, and minimize unnecessary prescription of antibiotics.
References
Mitchell MS, Sorrentino A, Centor RM. Adolescent pharyngitis: a review of bacterial causes. Clin Pediatr. 2011;50(12):1091–5.
Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, et al. Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin Microbiol Rev. 2014;27(2):264–301.
Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):e86–102.
Nasirian H, TarvijEslami S, Matini E, Bayesh S, Omaraee Y. A clinical decision rule for streptococcal pharyngitis management: an update. J Lab Physicians. 2017;9(2):116–20.
Bisno AL, Gerber MA, Gwaltney JJM, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis. 2002;35(2):113–25.
Martin JM. The mysteries of streptococcal pharyngitis. Curr Treat Opt Pediatr. 2015;1(2):180–9.
Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev. 2004;17(3):571–80.
Tanz RR, Gerber MA, Kabat W, Rippe J, Seshadri R, Shulman ST. Performance of a rapid antigen-detection test and throat culture in community pediatric offices: implications for management of pharyngitis. Pediatrics. 2009;123(2):437–44.
Stewart EH, Davis B, Clemans-Taylor BL, Littenberg B, Estrada CA, Centor RM. Rapid antigen group A Streptococcus test to diagnose pharyngitis: a systematic review and meta-analysis. PLoS One. 2014;9(11):e111727.
Pritt BS, Patel R, Kirn TJ, Thomson RB. Point-counterpoint: a nucleic acid amplification test for Streptococcus pyogenes should replace antigen detection and culture for detection of bacterial pharyngitis. J Clin Microbiol. 2016;54(10):2413–9.
Eboigbodin KE, Hoser MJ. Multiplex strand invasion based amplification (mSIBA) assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. Sci Rep. 2016;6:20487.
Hoser MJ, Mansukoski HK, Morrical SW, Eboigbodin KE. Strand invasion based amplification (SIBA®): a novel isothermal DNA amplification technology demonstrating high specificity and sensitivity for a single molecule of target analyte. PLoS One. 2014;9(11):e112656.
Eboigbodin K, Filén S, Ojalehto T, Brummer M, Elf S, Pousi K, et al. Reverse transcription strand invasion based amplification (RT-SIBA): a method for rapid detection of influenza A and B. Appl Microbiol Biotechnol. 2016;100(12):5559–67.
Formosa T, Alberts BM. Purification and characterization of the T4 bacteriophage uvsX protein. J Biol Chem. 1986;261(13):6107–18.
Kodani M, Yang G, Conklin LM, Travis TC, Whitney CG, Anderson LJ, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49(6):2175–82.
Eboigbodin KE, Brummer M, Ojalehto T, Hoser M. Rapid molecular diagnostic test for Zika virus with low demands on sample preparation and instrumentation. Diagn Microbiol Infect Dis. 2016;86(4):369–71.
Eboigbodin KE, Moilanen K, Elf S, Hoser M. Rapid and sensitive real-time assay for the detection of respiratory syncytial virus using RT-SIBA®. BMC Infect Dis. 2017;17(1):134.
Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors—occurrence, properties and removal. J Appl Microbiol. 2012;113(5):1014–26.
Al-Soud WA, Rådström P. Purification and characterization of PCR-inhibitory components in blood cells. J Clin Microbiol. 2001;39(2):485–93.
Kim S, Labbe RG, Ryu S. Inhibitory effects of collagen on the PCR for detection of Clostridium perfringens. Appl Environ Microbiol. 2000;66(3):1213–5.
Author information
Authors and Affiliations
Contributions
KE conceived the study. SE, JO, SH, PA, and KE performed the experiments, analyzed the results, and wrote and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
SE, JO, SH, PA, and KE are employees of Orion Diagnostica Oy. All SIBA patents and patent applications are owned by Orion Diagnostica Oy. KE and SE are named as inventors on patents and patent applications.
Funding
This work is funded by Orion Diagnostica Oy and Salwe Research program for GET IT DONE (Finnish funding agency for technology and innovation Grant 534/14).
Ethical approval and informed consent
Clinical specimens were acquired and used in accordance with Discovery Life Sciences Biobank Bioethics Policy.
Rights and permissions
About this article
Cite this article
Elf, S., Olli, J., Hirvonen, S. et al. Molecular Detection of Streptococcus pyogenes by Strand Invasion Based Amplification Assay. Mol Diagn Ther 22, 595–602 (2018). https://doi.org/10.1007/s40291-018-0346-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-018-0346-8