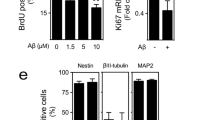
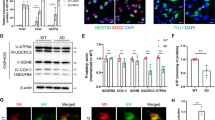
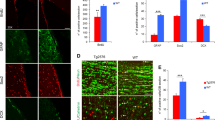
Abstract
Adult neurogenesis defects have been demonstrated in the brains of Alzheimer’s disease (AD) patients. The neurogenesis impairment is an early critical event in the course of familiar AD (FAD) associated with neuronal loss. It was suggested that neurologic dysfunction in AD may be caused by impaired functioning of hippocampal neural stem cells (NSCs). Multiple metabolic and structural abnormalities in neural mitochondria have long been suspected to play a critical role in AD pathophysiology. We hypothesize that the cause of such abnormalities could be defective elimination of damaged mitochondria. In the present study, we evaluated mitophagy efficacy in a cellular AD model, hiPSC-derived NSCs harboring the FAD-associated PS1 M146L mutation. We found several mitochondrial respiratory chain defects such as lower expression levels of cytochrome c oxidase (complex IV), cytochrome c reductase (complex III), succinate dehydrogenase (complex II), NADH:CoQ reductase (complex I), and also ATP synthase (complex V), most of which had been previously associated with AD. The mitochondrial network morphology and abundance in these cells was aberrant. This was associated with a marked mitophagy failure stemming from autophagy induction blockage, and deregulation of the expression of proteins involved in mitochondrial dynamics. We show that treating these cells with autophagy-stimulating drug bexarotene restored autophagy and compensated mitochondrial anomalies in PS1 M146L NSCs, by enhancing the clearance of mitochondria. Our data support the hypothesis that pharmacologically induced mitophagy enhancement is a relevant and novel therapeutic strategy for the treatment of AD.







Similar content being viewed by others
Abbreviations
- (AD):
-
Alzheimer’s disease
- (Aβ):
-
amyloid beta
- (APP):
-
amyloid beta (A4) precursor protein
- (AVs):
-
autophagic vacuoles
- (Baf A1):
-
bafilomycin A1
- (CCCP):
-
carbonyl cyanide m-chlorophenylhydrazone
- (DLP1):
-
dynamin-like protein 1
- (FAD):
-
familial Alzheimer’s disease
- (GAPDH):
-
glyceraldehyde-3-phosphate dehydrogenase
- (iPSC):
-
induced pluripotent stem cells
- HAR:
-
hexaammineruthenium (III)
- (LAMP1):
-
lysosomal-associated membrane protein 1
- (MAP1LC3/LC3):
-
microtubule-associated protein 1 light chain 3
- (mtDNA):
-
mitochondrial DNA
- (MFN1):
-
mitofusin 1
- (MFN2):
-
mitofusin 2
- (NPCs):
-
neural progenitor cells
- (OPA1):
-
optic atrophy 1
- (OPTN):
-
optineurin
- (OXPHOS):
-
oxidative phosphorylation system
- (PSEN):
-
presenilin
- (PINK1):
-
PTEN-induced putative kinase 1
- (ROS):
-
reactive oxygen species
- (RXR):
-
retinoid X receptor
- (TFEB):
-
transcription factor EB
- (TOMM20):
-
translocase of outer mitochondrial membrane 20 homolog
References
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Holtzman DM et al (2011) Mapping the road forward in Alzheimer’s disease. Sci Transl Med 3(114):114ps48
Klionsky DJ et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1):1–222
Ryan NS, Rossor MN (2010) Correlating familial Alzheimer’s disease gene mutations with clinical phenotype. Biomark Med 4(1):99–112
Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G et al (1996) Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron 17(5):1005–1013
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132(4):645–660
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6:85
Demars M et al (2010) Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res 88(10):2103–2117
Li B et al (2008) Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol 67(1):78–84
Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL et al (1997) Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 387(6630):288–292
Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S (1997) Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 89(4):629–639
Chen Q, Nakajima A, Choi SH, Xiong X, Sisodia SS, Tang YP (2008) Adult neurogenesis is functionally associated with AD-like neurodegeneration. Neurobiol Dis 29(2):316–326
Haughey NJ et al (2002) Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem 83(6):1509–1524
Zhang C et al (2007) Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol 204(1):77–87
Lazarov O, Marr RA (2010) Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol 223(2):267–281
Papa S et al (2004) Respiratory complex I in brain development and genetic disease. Neurochem Res 29(3):547–560
Calingasan NY et al (2008) Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases. Neuroscience 153(4):986–996
Kathleen Baxter K et al (2009) The neurogenic basic helix-loop-helix transcription factor NeuroD6 concomitantly increases mitochondrial mass and regulates cytoskeletal organization in the early stages of neuronal differentiation. ASN Neuro 1(4):195–211
Kirby DM, Rennie KJ, Smulders-Srinivasan TK, Acin-Perez R, Whittington M, Enriquez JA, Trevelyan AJ, Turnbull DM et al (2009) Transmitochondrial embryonic stem cells containing pathogenic mtDNA mutations are compromised in neuronal differentiation. Cell Prolif 42(4):413–424
Voloboueva LA, Giffard RG (2011) Inflammation, mitochondria, and the inhibition of adult neurogenesis. J Neurosci Res 89(12):1989–1996
Khacho M et al (2016) Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell 19(2):232–247
Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA (2010) Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology 59(4–5):290–294
Lemasters JJ (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 8(1):3–5
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12(1):9–14
Narendra DP et al (2010) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8(1):e1000298
Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RLA, Kim J, May J, Tocilescu MA et al (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A 107(1):378–383
Geisler S et al (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12(2):119–131
Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AHV, Taanman JW (2010) Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19(24):4861–4870
Bjorkoy G et al (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171(4):603–614
Martin-Maestro P et al (2016) PARK2 enhancement is able to compensate mitophagy alterations found in sporadic Alzheimer’s disease. Hum Mol Genet 25(4):792–806
Martin-Maestro P et al (2017) Mitophagy failure in fibroblasts and iPSC-derived neurons of Alzheimer’s disease-associated presenilin 1 mutation. Front Mol Neurosci 10:291
Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI et al (2007) Increased autophagic degradation of mitochondria in Alzheimer disease. Autophagy 3(6):614–615
Moreira PI et al (2007) Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol 66(6):525–532
Sodhi RK, Singh N (2014) Retinoids as potential targets for Alzheimer’s disease. Pharmacol Biochem Behav 120:117–123
Henney JE (2000) From the Food and Drug Administration. JAMA 283(9):1131
Abba MC, Hu Y, Levy CC, Gaddis S, Kittrell FS, Zhang Y, Hill J, Bissonnette RP et al (2008) Transcriptomic signature of bexarotene (rexinoid LGD1069) on mammary gland from three transgenic mouse mammary cancer models. BMC Med Genet 1:40
McFarland K, Spalding TA, Hubbard D, Ma JN, Olsson R, Burstein ES (2013) Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson's disease. ACS Chem Neurosci 4(11):1430–1438
Riancho J et al (2015) Neuroprotective effect of bexarotene in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Front Cell Neurosci 9:250
Bomben V et al (2014) Bexarotene reduces network excitability in models of Alzheimer’s disease and epilepsy. Neurobiol Aging 35(9):2091–2095
Cramer PE et al (2012) ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science 335(6075):1503–1506
Moutinho M, Landreth GE (2017) Therapeutic potential of nuclear receptor agonists in Alzheimer’s disease. J Lipid Res 58(10):1937–1949
Cummings JL et al (2016) Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer's disease. Alzheimers Res Ther 8:4
Pierrot N, Lhommel R, Quenon L, Hanseeuw B, Dricot L, Sindic C, Maloteaux JM, Octavea JN et al (2016) Targretin improves cognitive and biological markers in a patient with Alzheimer’s disease. J Alzheimers Dis 49(2):271–276
Boehm-Cagan A, Michaelson DM (2014) Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J Neurosci 34(21):7293–7301
Sproul AA et al (2014) Characterization and molecular profiling of PSEN1 familial Alzheimer’s disease iPSC-derived neural progenitors. PLoS One 9(1):e84547
Paquet D et al (2016) Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533(7601):125–129
Rubinsztein DC, Cuervo AM, Ravikumar B, Sarkar S, Korolchuk VI, Kaushik S, Klionsky DJ (2009) In search of an “autophagomometer”. Autophagy 5(5):585–589
Malik AN et al (2011) Mitochondrial DNA as a non-invasive biomarker: accurate quantification using real time quantitative PCR without co-amplification of pseudogenes and dilution bias. Biochem Biophys Res Commun 412(1):1–7
Sled VD, Vinogradov AD (1993) Kinetics of the mitochondrial NADH-ubiquinone oxidoreductase interaction with hexammineruthenium(III). Biochim Biophys Acta 1141(2–3):262–268
Chiang MC, Nicol CJ, Cheng YC, Lin KH, Yen CH, Lin CH (2016) Rosiglitazone activation of PPARgamma-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced mitochondrial dysfunction and oxidative stress. Neurobiol Aging 40:181–190
Manczak M, Park BS, Jung Y, Reddy PH (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. NeuroMolecular Med 5(2):147–162
Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H et al (2009) Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A 106(47):20057–20062
Stepanova A, Kahl A, Konrad C, ten V, Starkov AS, Galkin A (2017) Reverse electron transfer results in a loss of flavin from mitochondrial complex I: potential mechanism for brain ischemia reperfusion injury. J Cereb Blood Flow Metab 37(12):3649–3658
Kahl A, Stepanova A, Konrad C, Anderson C, Manfredi G, Zhou P, Iadecola C, Galkin A (2018) Critical role of flavin and glutathione in complex I-mediated bioenergetic failure in brain ischemia/reperfusion injury. Stroke 49(5):1223–1231
Napolitano G, Ballabio A (2016) TFEB at a glance. J Cell Sci 129(13):2475–2481
Puertollano R, Ferguson SM, Brugarolas J, Ballabio A (2018) The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J 37(11):e98804
Kim J et al (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141
Lee JH et al (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141(7):1146–1158
Ryan TA, Tumbarello DA (2018) Optineurin: a coordinator of membrane-associated cargo trafficking and autophagy. Front Immunol 9:1024
Narendra DP, Youle RJ (2011) Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid Redox Signal 14(10):1929–1938
Ni HM, Williams JA, Ding WX (2015) Mitochondrial dynamics and mitochondrial quality control. Redox Biol 4:6–13
Loson OC et al (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell 24(5):659–667
Palmer CS et al (2011) MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep 12(6):565–573
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29(28):9090–9103
Wang X, Su B, Fujioka H, Zhu X (2008) Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol 173(2):470–482
Martin-Maestro P et al (2017) Slower dynamics and aged mitochondria in sporadic Alzheimer’s disease. Oxidative Med Cell Longev 2017:9302761
Smirnova E, Griparic L, Shurland DL, van der Bliek A (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12(8):2245–2256
Valente AJ, Maddalena LA, Robb EL, Moradi F, Stuart JA (2017) A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture. Acta Histochem 119(3):315–326
Xia D, Watanabe H, Wu B, Lee SH, Li Y, Tsvetkov E, Bolshakov VY, Shen J et al (2015) Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron 85(5):967–981
Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Rao BSS, Chattarji S, Kelleher RJ III et al (2004) Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42(1):23–36
Wines-Samuelson M, Schulte EC, Smith MJ, Aoki C, Liu X, Kelleher RJ, Shen J (2010) Characterization of age-dependent and progressive cortical neuronal degeneration in presenilin conditional mutant mice. PLoS One 5(4):e10195
Veeraraghavalu K, Choi S, Zhang X, Sisodia SS (2013) Endogenous expression of FAD-linked PS1 impairs proliferation, neuronal differentiation and survival of adult hippocampal progenitors. Mol Neurodegener 8:41
Sherrington R et al (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375(6534):754–760
Bruni AC et al (2010) Worldwide distribution of PSEN1 Met146Leu mutation: a large variability for a founder mutation. Neurology 74(10):798–806
Page RM, Baumann K, Tomioka M, Pérez-Revuelta BI, Fukumori A, Jacobsen H, Flohr A, Luebbers T et al (2008) Generation of Abeta38 and Abeta42 is independently and differentially affected by familial Alzheimer disease-associated presenilin mutations and gamma-secretase modulation. J Biol Chem 283(2):677–683
Kuusisto E, Suuronen T, Salminen A (2001) Ubiquitin-binding protein p62 expression is induced during apoptosis and proteasomal inhibition in neuronal cells. Biochem Biophys Res Commun 280(1):223–228
Kuusisto E, Salminen A, Alafuzoff I (2002) Early accumulation of p62 in neurofibrillary tangles in Alzheimer's disease: possible role in tangle formation. Neuropathol Appl Neurobiol 28(3):228–237
Fernandez-Mosquera L et al (2017) Acute and chronic mitochondrial respiratory chain deficiency differentially regulate lysosomal biogenesis. Sci Rep 7:45076
Tanaka A (2010) Parkin-mediated selective mitochondrial autophagy, mitophagy: Parkin purges damaged organelles from the vital mitochondrial network. FEBS Lett 584(7):1386–1392
Lonskaya I, Shekoyan AR, Hebron ML, Desforges N, Algarzae NK, Moussa CE (2013) Diminished parkin solubility and co-localization with intraneuronal amyloid-beta are associated with autophagic defects in Alzheimer's disease. J Alzheimers Dis 33(1):231–247
Witte ME, Bol JGJM, Gerritsen WH, Valk P, Drukarch B, Horssen J, Wilhelmus MMM (2009) Parkinson's disease-associated parkin colocalizes with Alzheimer’s disease and multiple sclerosis brain lesions. Neurobiol Dis 36(3):445–452
Wang L et al (2016) Synaptosomal mitochondrial dysfunction in 5xFAD mouse model of Alzheimer’s disease. PLoS One 11(3):e0150441
Fang EF et al (2019) Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22(3):401–412
Ye X et al (2015) Parkin-mediated mitophagy in mutant hAPP neurons and Alzheimer’s disease patient brains. Hum Mol Genet 24(10):2938–2951
Garcia-Escudero V et al (2013) Deconstructing mitochondrial dysfunction in Alzheimer disease. Oxidative Med Cell Longev 2013:162152
Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X (2008) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 105(49):19318–19323
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9(7):505–518
Veeraraghavalu K, Zhang C, Miller S, Hefendehl JK, Rajapaksha TW, Ulrich J, Jucker M, Holtzman DM et al (2013) Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science 340(6135):924–92f
Ulrich JD, Burchett JM, Restivo JL, Schuler DR, Verghese PB, Mahan TE, Landreth GE, Castellano JM et al (2013) In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Mol Neurodegener 8(8):13
Mandrekar-Colucci S, Landreth GE (2011) Nuclear receptors as therapeutic targets for Alzheimer’s disease. Expert Opin Ther Targets 15(9):1085–1097
Tesseur I et al (2013) Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science 340(6135):924-e
Fitz NF, Cronican AA, Lefterov I, Koldamova R (2013) Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science 340(6135):924–92c
Huuskonen MT et al (2016) Bexarotene targets autophagy and is protective against thromboembolic stroke in aged mice with tauopathy. Sci Rep 6:33176
Dickey AS, Sanchez DN, Arreola M, Sampat KR, Fan W, Arbez N, Akimov S, van Kanegan MJ et al (2017) PPARdelta activation by bexarotene promotes neuroprotection by restoring bioenergetic and quality control homeostasis. Sci Transl Med 9(419):eaal2332
Malm T, Mariani M, Donovan LJ, Neilson L, Landreth GE (2015) Activation of the nuclear receptor PPARdelta is neuroprotective in a transgenic mouse model of Alzheimer’s disease through inhibition of inflammation. J Neuroinflammation 12:7
Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT (2012) PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci 32(48):17321–17331
Mandrekar-Colucci S, Karlo JC, Landreth GE (2012) Mechanisms underlying the rapid peroxisome proliferator-activated receptor-gamma-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J Neurosci 32(30):10117–10128
Acknowledgments
We would like to thank Marc Tessier-Lavigne for supporting the generation of cell lines, Matt Zimmer for performing FACS on GFP/RFP double-transfected cells to help generate PS1 M146L knockin iPSCs, Brian Campos for technical assistance in screening candidate clones, and Ana Sevilla for performing iPSC immunostaining characterization.
Funding
This work was supported by the NIA Grant P01AG014930 (A.A.S. and S.N.).
Author information
Authors and Affiliations
Contributions
PMM designed, performed, analyzed, and interpreted data as well as wrote the manuscript; AS, HM, DP, MTL CLM, and SN generated hiPSC-derived NSCs harboring the FAD-associated mutation. MG performed OXPHOS studies. AS and AAS contributed to data interpretation and manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Human subject research at the New York Stem Cell Foundation was performed in accordance with applicable federal and state regulations, as well as with guidelines established by the National Institutes of Health (NIH), National Academy of Sciences (NAS), and International Society for Stem Cell Research (ISSCR). It was also fully compliant with standards outlined in the Health Insurance Portability and Accountability Act (HIPAA) and in the Office for Human Research Protections (OHRP) recommendations.
Consent for Publication
All authors declare their consent for publication of this manuscript.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 2682 kb)
Rights and permissions
About this article
Cite this article
Martín-Maestro, P., Sproul, A., Martinez, H. et al. Autophagy Induction by Bexarotene Promotes Mitophagy in Presenilin 1 Familial Alzheimer’s Disease iPSC-Derived Neural Stem Cells. Mol Neurobiol 56, 8220–8236 (2019). https://doi.org/10.1007/s12035-019-01665-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01665-y