Root traits in Crambe abyssinica Hochst and Raphanus sativus L. plants are associated with differential tolerance to water deficit and post-stress recovery
- Published
- Accepted
- Received
- Academic Editor
- Efi Levizou
- Subject Areas
- Agricultural Science, Plant Science
- Keywords
- Drought, Oilseed, Gas exchange, Root morphology, Drought tolerance
- Copyright
- © 2022 de Freitas Moura et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Root traits in Crambe abyssinica Hochst and Raphanus sativus L. plants are associated with differential tolerance to water deficit and post-stress recovery. PeerJ 10:e13595 https://doi.org/10.7717/peerj.13595
Abstract
Background
Previous studies have shown that Crambe abyssinica and Raphanus sativus are physiologically tolerant to water deficits; however, there is a lack of information on the mechanisms responsible for their tolerance regarding root morphological characteristics. This study aimed to characterize morphological changes in the root system of C. abyssinica and R. sativus subjected water deficit, as well as to identify the responses that improve tolerance and post-stress recovery capacity of these plants.
Methods
Independent experiments for each specieswere performed in a controlled greenhouse, where plants were randomly set in a randomized block design with five replicates. Plants of C. abyssinica and R. sativus were cultivated in pots and exposed to well-watered treatment (WW; 90% water holding capacity–WHC of the substrate) or water deficit (WD; 40% WHC) conditions, at 28 days after planting. The plants were kept under WD for 7, 14, or 21 days with rehydration soon after each episode of water deficit. Assessment of water relations, biomass allocation, leaf and root system morphological characteristics and gas exchange were performed after each period of water deficit and 48 h after rehydration.
Results
The water deficit reduced the water status of both species, and morphological and biomass allocation were not recovered after rehydration. Photosynthesis of C. abyssinica decreased with prolonged water deficit, which was also not recovered after rehydration. In R. sativus, photosynthesis was not altered by WD for 21 days, and a higher WUE was recorded. Root morphology of R. sativus was mainly affected at 14 days of WD, while the traits related to very fine roots increased at 21 days of WD, when compared to WW plants. Thus, R. sativus has shown greater tolerance to water deficits mainly due to the presence of very fine roots throughout the period of stress, when compared to C. abyssinica in which the fine roots predominated.
Introduction
Crambe abyssinica Hochst and Raphanus sativus L. var. oleiferus are non-food crops considered potential oil source for the chemical industry (Vasconcelos et al., 2018; Samarappuli et al., 2020). The seeds of these species contain 20% (R. sativus) to 60% (C. abyssinica) of erucic acid in oil content, that makes them raw materials with high manufacturing value for biodiesel production (Donadon et al., 2015; Oliveira et al., 2015; Silveira et al., 2019). The use of renewable energy sources constitutes an environmental advantage by reducing the emission of polluting gases (Zhu, 2016).
As observed in other crops, grain yields of C. abyssinica and R. sativus are subject to the severity of water constraints (Boiago et al., 2018; Stagnari et al., 2018). The physiological tolerance of these plants to drought stress has already been observed (de Moura et al., 2018), and longer periods of water deficit may cause significant reductions in plant growth and development (Basu et al., 2016; Abid et al., 2018), and consequently, in grain yield (Rivas et al., 2016). Plants ability to maintain its physiological functions during and after water deficit relies on adaptive mechanisms of the aerial part and/or root system (Chen et al., 2016; Bristiel et al., 2019). The severity and duration of the stresses are also important factors (Basu et al., 2016).
Physiological and biochemical responses related to drought tolerance in the shoot of plants have already been widely reported, where (Osmolovskaya et al., 2018; Zhu et al., 2020; Dhansu et al., 2021; Mthembu et al., 2022) most of these responses are found to be directly related to the ability of the root system to efficiently obtain water and nutrients. However, when it comes to adaptive mechanisms involving root morphology, there are few studies related to drought tolerance compared to plant shoot responses (Wasaya et al., 2018). The study of the roots of plants grown in soil is still challenging, either because of the technical difficulties to evaluate this system in the plant in vivo, or because of the limitations in collecting the entire root system (Li et al., 2021). Nevertheless, understanding the roles of root system architecture and functions are crucial for choosing the best adapted species for cultivation in regions subjected to drought (Lynch, 2015).
In addition to the crucial role of water and nutrient absorption, roots are organs that signal drought in the soil (Vadez, 2014; Takahashi et al., 2020). The drought avoidance strategy in plants maintain an adequate water status through continuous water absorption. Usually this can be noticed on a deeper root system, with greater soil exploration capacity (Belachew et al., 2018; Katuwal, Schwartz & Jespersen, 2020). This response increases the efficiency of water and nutrient used by plants, reducing the impact of stresses. Thus, a robust and well-developed root system can determine survival and productivity of crops under limiting water conditions (Koevoets et al., 2016; Ramamoorthy et al., 2017).
Roots traits such specific length, specific surface area, root area-to-leaf area ratio, tissue density, length density, and a smaller root diameter are attributes of the root system involved in adaptation to water deficit (Comas et al., 2013; Wasaya et al., 2018; Li et al., 2021). In this regard, recent studies have focused attention on traits of the root system as targets for breeding strategies for greater drought tolerance in crops (White, 2019; Xiong et al., 2021; Maqbool et al., 2022).
Several authors claim that the root system bestow drought tolerance to C. abyssinica and R. sativus (Falasca et al., 2010; Cremonez et al., 2013; Dias et al., 2015; Bassegio et al., 2016; Zanetti et al., 2016), but few studies have identified which root traits are involved in this response in both species. In this study, we tested two hypotheses: (i) adaptive adjustments in the root morphology of C. abyssinica and R. sativus increase the tolerance to water deficit and the capacity of plants to recover after stress; and (ii) whether the proportion of thinner fine roots is decisive in differentiating the level of tolerance to water deficit between these species. Thus, this study aimed to characterize morphological changes in the root system of C. abyssinica and R. sativus subjected to water deficit, as well as to identify the responses which may improve the tolerance and post-stress recovery capacity of these plants.
Material and Methods
Plant material and growth conditions
Independent experiments were performed at the Laboratory of Ecophysiology and Plant Productivity at the Goiano Federal Institute–Rio Verde campus, Goiás, Brazil (17°48′07.9″S 50°54′20.7″W), from March to May 2018 for each species in a greenhouse with controlled temperature (~26 °C) and relative humidity (51–75%). Plants of Crambe abyssinica Hochst (FMS Brilhante, 2017/2017 crop) and Raphanus sativus L. var. oleiferus Mertzg (IPR 116, 2017/2017 crop) were cultivated in 4.65 dm3 plastic pots (14.0 high diameter × 10.2 base diameter × 35.5 cm height) filled with 4.5 kg of a mixture of Red Latosol soil (LVdf) and sand (2:1). The substrate had the following chemical characteristics: P – 0.7 mg dm−3; K – 13.0 mg dm−3; Ca – 1.54 cmolc dm−3; Mg – 0.22 cmolc dm−3; Al – 0.05 cmolc dm−3; H+Al – 1.3 cmolc dm−3; S – 3.5 mg dm−3; B – 0.8 mg dm−3; Cu – 1.0 mg dm−3; Fe – 37.8 mg dm−3; Mn – 13.2 mg dm−3; Zn – 0.1 mg dm−3; Na – 6.0 mg dm−3; pH CaCl2 – 5.6; SB – 58%; cation exchange capacity – 3.1 cmolc dm−3; organic matter – 10.9 g dm−3. The total substrate (0.60 m3) was fertilized with 82.8 g of formulated NPK 4 - 14 - 08 and 2.4 g of filler dolomitic limestone to increase base saturation to 60%. The water holding capacity (WHC) of the substrate was determined through the saturation of the pots and the moisture was monitored daily by the gravimetric method.
A total of 28 days after planting, plants of C. abyssinica and R. sativus having three of four pairs of expanded leaves were exposed to well-watered conditions (WW; 90% WHC) and water deficit (WD; 40% WHC) conditions. The plants were kept under WD for 7, 14, and 21 days with rehydration for 48 h soon after each episode of water deficit. Treatments consisted of well-watered plants at 7 (WW7), 14 (WW14) and 21 (WW21) days; water deficit for 7 (WD7), 14 (WD14) and 21 (WD21) days; and rehydrated plants after WD for 7 (RH7), 14 (RH14) and 21 (RH21) days.
The experiments were performed with a randomized block design including five replicates. Each experimental unit consisted of one plant per pot.
Water relations
Predawn leaf water potential (ψw) was measured between 04:00 and 06:00 am using a Scholander pressure chamber (model 3005-1412, Soilmoisture Equipment Corp., Goleta, CA, USA). Leaf osmotic potential (ψsl) and root osmotic potential (ψsr) were evaluated using a vapor pressure osmometer (VAPRO 5600, VAPRO®, Wescor, UT, USA) according to Pierre & Arce (2012). Osmotic potential values were calculated using the Van’t Hoff equation: (ψs = −R × T × Cs, where R is the universal gas constant (0.08205 L atm mol−1 K−1), T is the temperature in °K (T °K = T °C + 273) and Cs the solute concentration (M), usually expressed in atmospheres and converted to MPa (0.987≈ 1 atm 0.1 MPa). Relative water content (RWC) was determined from fresh matter (FM), turgid matter (TM) and dry matter (DM) data obtained by weighing leaf discs according to Barrs & Weatherley (1962), and calculated as RWC (%) = (FM − DM)/(TM − DM) × 100.
Morphological analysis and biomass allocation
Plants were measured to determine the plant height (PH, cm), stem diameter (SD, mm), nodes number (NN), leaf number (LN), and the main root length (RL, cm). The leaf area (LA, m2) was calculated using the ImageJ software (Image Processing and Analysis in Java, v. 1.52d, USA). Leaves, stem and roots were separated into paper bags and dried in an air circulation oven at 65 °C until constant weight to obtain the leaf dry matter (LDM, g), stem dry matter (StDM, g) and root dry matter (MSR, g). Shoot dry matter (SDM = LDM + StDM), total dry matter (TDM = SDM + RDM), specific leaf area (SLA), leaf area ratio (LAR) and RDM/SDM were calculated.
The root system was evaluated with digital images obtained by digital camera (6MP; 3264 × 1836 px) using the Safira v1.1 software (Fiber and Root Analysis System; Embrapa Agricultural Instrumentation, São Paulo, Brazil). The roots were classified into three diameter classes: very fine roots (Ø < 0.5 mm), fine roots (0.5 < Ø < 2.0 mm) and large roots (Ø > 2.0 mm), according to the criterion proposed by Böhm (1979). From the data obtained by image processing, the morphological characteristics were determined: mean root diameter (MRD, mm), very fine root length (VFRL, cm), fine root length (FRL, cm), large root length (LRL, cm), total root length (TRL, cm), very fine root surface area (VFRA, cm2), fine root surface area (FRA, cm2), large root area (LRA, cm2), total root surface area (TRA, cm2), very fine root volume (VFRV, cm3), fine root volume (FRV, cm3), large root volume (LRV, cm3), total root volume (TRV, cm3), TRV/SDM ratio (cm g−1), specific root length (SRL = TRL/RDM, cm g−1), root thickness, (RT = TRV/VTR, cm cm−3), root tissue density (RTD = RDM/TRV, g cm−3), total root area and leaf area ratio (TRA/LA, m2 m−2), specific surface area (SSA = TRA/RDM, cm2 g−1) and root length density (RLD = TRL/Vsoil, cm cm−3).
Gas exchange
Gas exchange analyses were carried out with an infrared gas analyzer (LI-6400XTR, Licor®, Lincoln, Nebraska, EUA). Photosynthetic rate (A, μmol CO2 m−2 s−1), stomatal conductance (gs, mol H2O m−2 s−1), transpiration (E, mmol H2O m−2 s−1) and the relation between internal and external CO2 concentration (Ci/Ca) were measured in fully expanded leaves in the middle third of the plant, obtained under constant photosynthetic photon flux density (PPFD, 1000 µmol photons m−2 s−1), atmospheric CO2 concentration (Ca) (~384 µmol mol−1), temperature (~26 °C), and relative humidity (~64%). From these data, the instantaneous water use efficiency (WUE = A/E) was calculated.
Statistical analysis
The data obtained were subjected to ANOVA (p ≤ 0.05) and treatment means were compared by the Scott-Knott clustering method (p ≤ 0.05) using the Analysis of Variance System software (SISVAR®, version 5.3). For multivariate analyses, data were scaled bg the means of mean-centering and scaled by the root-mean-square , using the scale function in R (R Core Team, 2021). Principal Component Analyses (PCAs) were modelled using the package pcaMethods (Stacklies et al., 2007) and graphs were generated using FactoMineR and factoextra packages, respectively (Kassambara & Mundt, 2020; Lê, Josse & Husson, 2008). Initial models were built using data from all period in order to assess an optimal number of Principal Components. By assessing the eigenvalues, the best number of Principal Components (PCs) was #4 for both species (Fig. S1). The R script and data are available at the public repository: https://github.com/candidosobrinhosa/MOURA_LMF_2022.
Results
Water relations
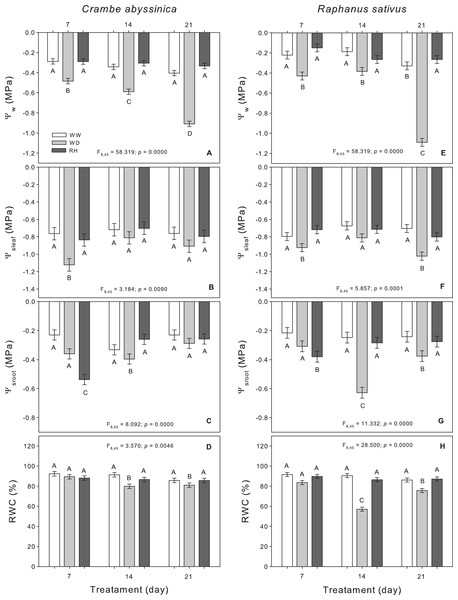
The water deficit decreased the Ψw in C. abyssinica, which was intensified according to the stress duration (Fig. 1A). The relative water content decreased with increasing in water deficit duration, but the values remained around 80% (Fig. 1D). With rehydration, all characteristics recovered to equal well-watered plants. The exception was the root osmotic potential which decreased in rehydrated plants after 7 days of stress (RH7) vs. WW-treated plants (WW7) and WD-treated plants (WD7) (Fig. 1C).
Figure 1: Water relations in Crambe abyssinica and Raphanus sativus.
Leaf water potential (Ψw), leaf osmotic potential (Ψsleaf) (B; F), root osmotic potential (Ψsroot) (C; G) and relative leaf water content (RWC) (D; H) in Crambe abyssinica (A–D) and Raphanus sativus (E–F) plants under well-watered (WW) and water deficit (WD) for 7, 14 or 21 days, and after rehydration (RH) condition. Bars represent means ± SE (n = 5). Means followed by different letters between treatments differ by the Scott-Knott test (p < 0.05).In general, as expected, the duration of water restriction reduced the water status of R. sativus plants, which were observed through leaf water potential, leaf osmotic potential, root osmotic potential, and relative water content at 21 days after the onset of water deficit (WD21). Upon rehydration, these characteristics reached values similar to those of the WW plants regardless of the evaluation time. On the other hand, root osmotic potential decreased after rehydration in 7-days WD-treated R. sativus plants (RH7) (Fig. 1G).
Morphological traits of the aerial part
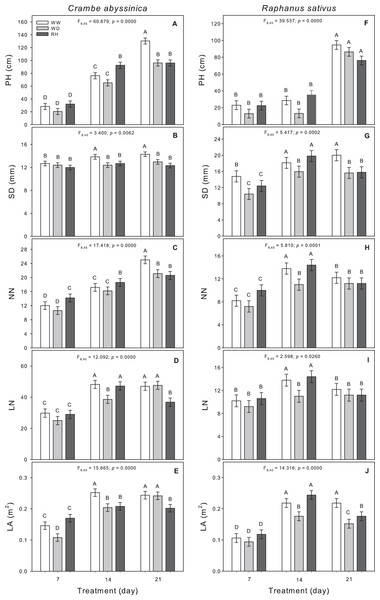
The prolonged duration of the water deficit inhibited the growth of C. abyssinica plants as seen by reductions in plant height, stem diameter, and node number (Fig. 2). After 7 (WD7) and 14 days (WD14) from the water deficit imposition, reductions in leaf area were observed (Fig. 2E), and the leaf number decreased only after the 14-day stress period (Fig. 2D). Upon rehydration, the C. abyssinica plants did not recover their morphological traits (Fig. 2).
Figure 2: Morphological traits in Crambe abyssinica and Raphanus sativus.
Plant height (PH) (A; F), stem diameter (SD) (B; G), node number (NN) (C; H), leaf number (LN) (D; I), and leaf area (LA) (E; J) in Crambe abyssinica (A–E) and Raphanus sativus (F–J) plants under well-watered (WW) and water deficit (WD) for 7, 14 or 21 days, and after rehydration (RH) condition. Bars represent means ± SE (n = 5). Means followed by different letters between treatments differ by the Scott-Knott test (p < 0.05).The 14-day water deficit caused reductions in stem diameter, node number, leaf number and leaf area in R. sativus plants (Figs. 2G–2J). The permanency of stress led to a reduction in stem diameter; but leaf area was maintained in the WD plants. After rehydration (RH21), the effects of the 21-day stress (WD21) on stem diameter and leaf area were not reversed (Figs. 2G and 2J).
Biomass allocation
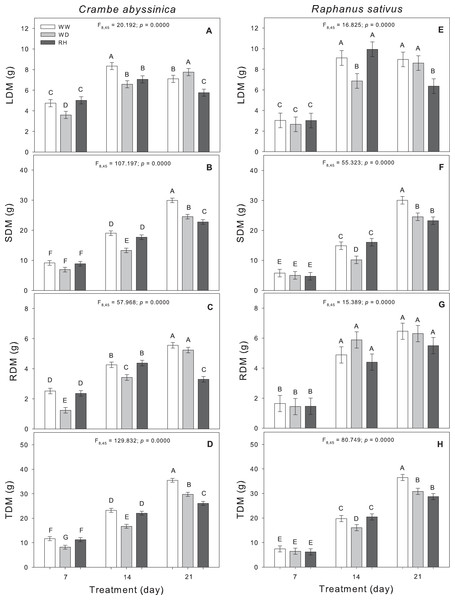
Changes in the biomass allocation of C. abyssinica plants were observed during the initial phase of stress. As the duration of water deficit increased, the shoot dry matter, and total dry matter were reduced in WD-treated plants (Fig. 3). Upon rehydration after a 21-day stress period (RH21), the dry matter of C. abyssinica was lower compared to WW plants (WW21) (Fig. 3).
Figure 3: Biomass allocation in Crambe abyssinica and Raphanus sativus.
Leaf dry matter (LDM) (A; E), shoot dry matter (SDM) (B; F), root dry matter (RDM) (C; G), and total dry matter (TDM) (D; H) in Crambe abyssinica (A–D) and Raphanus sativus (E–H) plants under well-watered (WW) and water deficit (WD) for 7, 14 or 21 days, and after rehydration (RH) condition. Bars represent means ± SE (n = 5). Means followed by different letters between treatments differ by the Scott-Knott test (p < 0.05).In R. sativus, the prolonged duration of the WD inhibited the allocation of biomass in leaves, shoots, and thus, reduced the total dry matter. Shoot and total dry matter of R. sativus did not recover in RH21 (Fig. 3).
Morphological traits of the root system
The water deficit caused changes in the architecture of the root system of C. abyssinica and R. sativus (Tables 1 and 2). In C. abyssinica at WD7 treatment there was an increase in the specific surface area (SSA) and very fine roots volume (VFRV) as well as in the rehydrated plants (RH7). Reductions in mean root diameter and increase in fine root length were observed after 14 days of stress. At 21 days, root depth decreased in C. abyssinica plants under water deficit. The mean root diameter, and fine and total root volume increased in the rehydrated plants after 14 days of water deficit (RH14). The root length, root surface area, and volume of large roots increased with rehydration after 21 days of water deficit (RH21) (Tables 1 and 2).
| TREAT | RL | MRD | VFRL | FRL | LRL | TRL | VFRA | FRA | LRA | TRA | VFRV | FRV | LRV | TRV | |
| Crambe abyssinica | |||||||||||||||
| 7 | WW | 50.8 A | 0.58 D | 266 A | 56.6 C | 2.2 C | 325 B | 44.3 B | 19.4 B | 1. 57 C | 65.3 B | 0.62 B | 0.61 C | 0.09 B | 1.31 D |
| WD | 50.3 A | 0.59 D | 301 A | 64.4 C | 2.4 C | 367 B | 53.8 A | 22.6 B | 1.79 C | 78.3 B | 0.77 A | 0.72 C | 0.08 B | 1.57 D | |
| RH | 51.2 A | 0.57 D | 297 A | 54.7 C | 2.3 C | 354 B | 51.8 A | 20.9 B | 1.77 C | 74.4 B | 0.71 A | 0.57 C | 0.08 B | 1.37 D | |
| 14 | WW | 54.1 A | 0.83 B | 0.00 B | 511 B | 5.9 A | 517 A | 0.00 C | 135 A | 3.92 A | 139 A | 0.00 C | 2.95 B | 0.19 A | 3.15 B |
| WD | 49.0 A | 0.72 C | 0.00 B | 668 A | 1.0 D | 669 A | 0.00 C | 158 A | 0.65 D | 158 A | 0.00 C | 3.10 B | 0.04 C | 3.13 B | |
| RH | 51.5 A | 0.91 A | 0.00 B | 503 B | 3.8 B | 507 A | 0.00 C | 150 A | 2.90 B | 152 A | 0.00 C | 3.61 A | 0.17 A | 3.78 A | |
| 21 | WW | 50.9 A | 0.70 C | 0.00 B | 596 A | 0.5 D | 597 A | 0.00 C | 133 A | 0.35 D | 133 A | 0.00 C | 2.35 B | 0.02 C | 2.37 C |
| WD | 43.7 B | 0.69 C | 0.00 B | 587 A | 0.5 D | 587 A | 0.00 C | 144 A | 0.32 D | 144 A | 0.00 C | 2.67 B | 0.02 C | 2.69 C | |
| RH | 43.2 B | 0.71 C | 0.00 B | 615 A | 1.8 C | 617 A | 0.00 C | 144 A | 1.23 C | 145 A | 0.00 C | 2.81 B | 0.07 B | 2.88 C | |
| F8,45 value | 2.512 | 36.040 | 101.139 | 58.585 | 27.693 | 10.469 | 94.992 | 46.208 | 27.224 | 14.141 | 92.530 | 38.835 | 24.669 | 3.356 | |
| p value | 0.0305 | 0.0003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0067 | |
| Raphanus sativus | |||||||||||||||
| 7 | WW | 39.1 B | 0.62 D | 401.9 C | 70.5 C | 3.2 D | 475.6 C | 71.1 D | 27.5 C | 2.59 C | 101 D | 1.32 F | 0.89 D | 0.15 D | 2.36 E |
| WD | 28.8 C | 0.67 C | 329.9 C | 77.3 C | 3.1 D | 410.4 C | 62.4 D | 31.2 C | 2.38 C | 96.0 D | 0.92 F | 1.03 D | 0.16 D | 2.10 E | |
| RH | 45.0 A | 0.58 D | 402.1 C | 165 A | 14 B | 580.9 C | 70.5 D | 66.1 B | 12.5 B | 149 D | 0.98 F | 2.18 C | 0.64 B | 3.81 D | |
| 14 | WW | 51.7 A | 0.79 B | 1,287 A | 157 A | 24 A | 1,468 A | 318 A | 77.3 A | 20.7 A | 416 A | 6.34 B | 3.49 B | 1.53 A | 11.4 B |
| WD | 48.2 A | 0.68 C | 1,243 A | 85.1 C | 8.1 C | 1,336 A | 243 B | 32.6 C | 6.38 C | 282 B | 3.99 C | 1.28 D | 0.35 C | 5.62 C | |
| RH | 48.1 A | 0.91 A | 1,220 A | 156 A | 5.5 D | 1,382 A | 344 A | 91.7 A | 5.05 C | 441 A | 7.74 A | 4.85 A | 0.66 B | 13.3 A | |
| 21 | WW | 49.3 A | 0.72 C | 640.9 B | 115 B | 5.3 D | 760.7 B | 170 C | 53.1 B | 5.27 C | 229 C | 2.81 D | 2.19 C | 0.41 C | 5.41 C |
| WD | 54.7 A | 0.66 C | 1,230 A | 147 A | 3.4 D | 1,381 A | 242 B | 61.4 B | 3.70 C | 307 B | 3.92 C | 2.53 C | 0.22 D | 6.68 C | |
| RH | 43.0 A | 0.68 C | 627.8 B | 80.2 C | 4.6 D | 712.6 B | 141 C | 36.2 C | 3.43 C | 180 C | 2.20 E | 1.44 D | 0.22 D | 3.87 D | |
| F8,45 value | 5.302 | 10.681 | 27.571 | 13.713 | 69.390 | 26.080 | 37.880 | 13.100 | 31.556 | 34.358 | 56.498 | 18.047 | 43.371 | 15.971 | |
| p value | 0.0003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
Notes:
Main root length (RL, cm), mean root diameter (MRD, mm), very fine root length (VFRL, cm), fine root length (FRL, cm), large root length (LRL, cm), total root length (TRL, cm), very fine root surface area (VFRA, cm2), fine root surface area (FRA, cm2), large root surface area (LRA, cm2), total root surface area (TRA, cm2), very fine root volume (VFRV, cm3), fine root volume (FRV, cm3), large root volume (LRV, cm3), and total root volume (TRV, cm3) in Crambe abyssinica and Raphanus sativus plants under well-watered (WW) and water deficit (WD) for 7, 14 or 21 days, and after rehydration (RH) condition. Very fine root (Ø < 0.5 mm), fine root (0.5 < Ø < 2.0 mm) and large root (Ø > 2.0 mm).
Means followed by different letters between treatments, in the columns, differ from each other by the Scott-Knott test (p < 0.05).
| DAT | TREAT | SLA | LAR | RDM/ SDM |
TRL/SDM | SRL | RT | RTD | TRA/LA | SSA | RLD |
| Crambe abyssinica | |||||||||||
| 7 | WW | 31.3 A | 12.7 B | 0.28 A | 35.7 C | 133 C | 256 A | 2.05 A | 0.04 B | 27.0 C | 0.15 B |
| WD | 29.8 A | 13.4 B | 0.18 C | 52.8 A | 307 A | 232 B | 0.79 B | 0.07 A | 65.5 A | 0.16 B | |
| RH | 33.9 A | 15.2 A | 0.26 A | 39.9 B | 151 C | 259 A | 1.76 A | 0.05 B | 31.7 C | 0.16 B | |
| 14 | WW | 30.6 A | 10.9 B | 0.23 B | 27.2 C | 122 C | 165 C | 1.38 B | 0.06 B | 32.7 C | 0.23 A |
| WD | 31.0 A | 12.3 B | 0.26 A | 51.5 A | 196 B | 215 B | 1.13 B | 0.08 A | 46.4 B | 0.30 A | |
| RH | 29.7 A | 9.53 C | 0.25 A | 29.0 C | 116 C | 134 C | 1.16 B | 0.07 A | 34.8 C | 0.23 A | |
| 21 | WW | 35.1 A | 6.93 C | 0.19 C | 20.2 C | 108 C | 256 A | 2.42 A | 0.05 B | 24.1 C | 0.27 A |
| WD | 31.3 A | 8.13 C | 0.21 B | 24.0 C | 114 C | 222 B | 2.02 A | 0.06 B | 27.8 C | 0.26 A | |
| RH | 35.2 A | 7.70 C | 0.15 C | 27.2 C | 189 B | 214 B | 1.15 B | 0.07 A | 44.3 B | 0.27 A | |
| F8,45 value | 1.712 | 14.647 | 9.786 | 10.687 | 14.158 | 12.528 | 8.379 | 3.913 | 12.599 | 10.662 | |
| p value | 0.1337 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0026 | <0.0001 | <0.0001 | |
| Raphanus sativus | |||||||||||
| 7 | WW | 37.5 A | 14.2 B | 0.29 B | 86.0 B | 292 B | 203 B | 0.71 C | 0.10 B | 62.4 B | 0.21 C |
| WD | 35.1 A | 13.9 B | 0.30 B | 88.3 B | 290 B | 195 B | 0.69 C | 0.12 B | 67.9 B | 0.18 C | |
| RH | 40.3 A | 19.1 A | 0.33 B | 129 A | 401 A | 153 C | 0.39 C | 0.13 B | 103 A | 0.26 C | |
| 14 | WW | 24.0 B | 11.1 C | 0.34 B | 101 B | 308 B | 130 C | 0.43 C | 0.20 A | 87.1 A | 0.65 A |
| WD | 25.9 B | 11.1 C | 0.57 A | 135 A | 255 B | 235 A | 1.08 B | 0.17 A | 52.7 B | 0.59 A | |
| RH | 24.9 B | 11.9 C | 0.28 B | 88.2 B | 314 B | 105 D | 0.34 C | 0.19 A | 100 A | 0.61 A | |
| 21 | WW | 24.7 B | 5.98 D | 0.22 B | 25.3 C | 125 C | 145 C | 1.28 A | 0.10 B | 37.8 C | 0.34 B |
| WD | 18.3 B | 4.91 D | 0.27 B | 57.7 C | 227 B | 210 B | 0.97 B | 0.20 A | 50.4 B | 0.61 A | |
| RH | 28.6 B | 6.25 D | 0.24 B | 30.7 C | 131 C | 184 B | 1.50 A | 0.10 B | 33.2 C | 0.32 B | |
| F8,45 value | 10.312 | 31.169 | 5.641 | 8.350 | 10.143 | 23.226 | 9.632 | 4.096 | 13.688 | 25.854 | |
| p value | <0.0001 | <0.0001 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0019 | <0.0001 | <0.0001 | |
Notes:
Specific leaf area (SLA, m2 kg−1), leaf area ratio (LAR, m2 kg−1), root/shoot dry matter ratio (RDM/SDM), total root length/shoot dry matter ratio (TDL/SDM, cm g−1), specific root length (SRL, cm g−1), root thickness (RT, cm cm−3), root tissue density (RTD, g cm−3), total root area ratio/leaf area (TRA/LA, m2 m−2), specific surface area (SSA, cm2 g−1), and root length density (RLD, cm cm−3) in Crambe abyssinica and Raphanus sativus plants under well-watered (WW) and water deficit (WD) for 7, 14 or 21 days, and after rehydration (RH) condition.
Means followed by different letters between treatments, in the columns, differ from each other by the Scott-Knott test (p < 0.05).
The water deficit reduced the mean root diameter at 7 (WD7) and 14 days (WD14) of water deficit in R. sativus plants. The length of fine and large roots as well as the surface area and volumes of very fine, fine, large, and total roots were reduced 14 days after the onset of water deficit (WD14). The length of very fine, fine, and total roots; surface area of very fine roots; surface area of total roots; and the volume of very fine roots increased at 21 days in plants under water deficit (WD21) (Table 1). After rehydration, the length, surface area, and volume of fine and large roots, as well as the total root volume increased in the rehydrated plants after 7 days of water deficit (RH7). During rehydration after 14 days of WD (RH14), there was an increase in the mean root diameter and volume of very fine, fine, and total roots, compared to WW plants. Rehydration after 21 days of stress (RH21) caused reductions in fine root length; fine root surface area; and very fine, fine, large, and total root volume, compared to WW plants (Table 1).
Proportion of roots by diameter classes in length, surface area, and volume
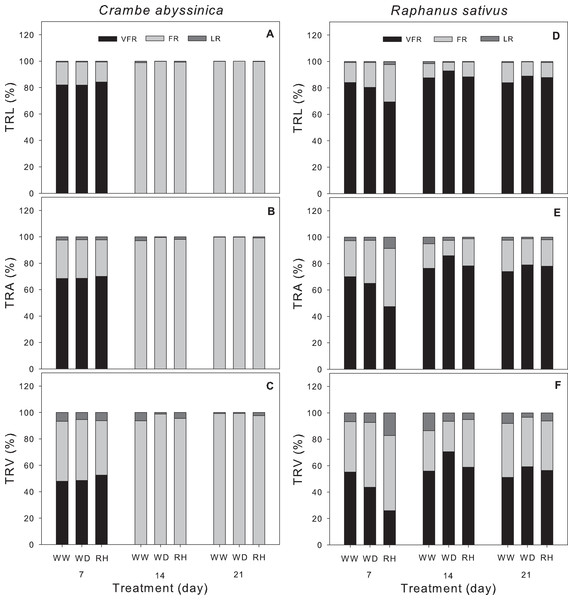
A higher proportion of fine roots was observed in C. abyssinica after 7 days, regardless of the treatment (Figs. 4A–4C). On the other hand, R. sativus showed a greater proportion of very fine roots vs. fine and large roots over all periods evaluated (Figs. 4D–4F). In this species, there was an increase in very fine roots under conditions of water deficit and rehydration at 14 and 21 days. Low proportions of large roots were observed in both species (Fig. 4).
Figure 4: Morphological traits of the root system in Crambe abyssinica and Raphanus sativus.
Proportion of very fine root (VFR), fine root (FR), and large roots (LR) in the total root length (TRL) (A; D), total root surface area (TRA) (B; E), and total root volume (TRV) (C; F) in Crambe abyssinica and Raphanus sativus plants under well-watered (WW) and water deficit (WD) for 7, 14 or 21 days, and after rehydration (RH) condition. Bars represent means (n = 5). Very fine root (Ø < 0.5 mm), fine root (0.5 < Ø < 2.0 mm) and large root (Ø > 2.0 mm).Morphological analyses of shoot and root system
In C. abyssinica, water deficits promoted an increase in the total root length/shoot dry matter ratio (TRL/SDM), specific root length, total root area/leaf area ratio (TRA/LA), and specific surface area. Reductions in root thickness and root tissue density were observed 7 days after the beginning of the WD period. There was an increase in the ratio RDM/SDM, TRL/SDM, specific root length, root thickness, TRA/LA, and specific surface area in a 14-day water deficit (WD14). The increase in the RDM/SDM ratio and the reduction in root thickness were observed at 21 days in plants under a water deficit. Upon rehydration after 14 days of stress, the RDM/SDM and TRA/LA ratios remained higher than the WW plants. The increase in the specific length of root, the TRA/LA, and the specific surface area as well as the reduction in the density of root tissue were observed in C. abyssinica rehydrated after 21 days of water deficit (Table 2).
In R. sativus, 14 days of water deficit increased the RDM/SDM ratio, TRL/SDM, root thickness, and root tissue density, but the specific surface area decreased in stressed plants. At 21 days of water deficit, there was an increase in the root-specific length, root thickness, TRA/LA, specific surface area, and root length density as well as a reduction in root tissue density. Rehydration after 7 days of water deficit led to an increase in the leaf area ratio, TRL/SDM, specific root length, and specific surface area compared to WW plants. The effect of stress on other root characteristics was reversed after rehydration (Table 2).
Gas exchange
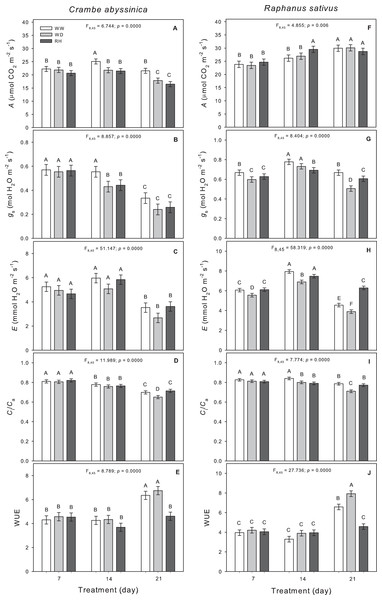
Reductions in the photosynthetic rate and Ci/Ca were observed in C. abyssinica plants as the water deficit persisted (Figs. 5A and 5D). The stomatal conductance decreased only in plants exposed to 14 days of water deficit (WW14) compared to WW plants (Fig. 5B). The reductions observed in A and gS were not reversed after plants rehydration (Figs. 5A and 5B).
Figure 5: Gas exchange in Crambe abyssinica and Raphanus sativus.
Photosynthetic rate (A) (A; F), stomatal conductance (gs) (B; G), transpiration rate (E) (C; H), relation between internal and external CO2 concentration (Ci/Ca) (D; I), and water use efficiency (WUE) (E; J) in Crambe abyssinica (A–E) and Raphanus sativus (F–J) plants under well-watered (WW) and water deficit (WD) for 7, 14 or 21 days, and after rehydration (RH) condition. Bars represent means ± SE (n = 5). Means followed by different letters between treatments differ by the Scott-Knott test (p < 0.05).In R. sativus, the water deficit caused reductions in gS, E, and Ci/Ca (Figs. 5G–5I), which in turn led to an increase in WUE at 21 days after WD imposition (Fig. 5J). Upon rehydration after a period of 14 days under water deficit, there was an increase in A but with lower gS in R. sativus plants (Figs. 5F and 5G). The gS was not fully recovered after the 21-day WD, but the E increased compared to WW plants (Figs. 5G and 5H).
Multivariate approach
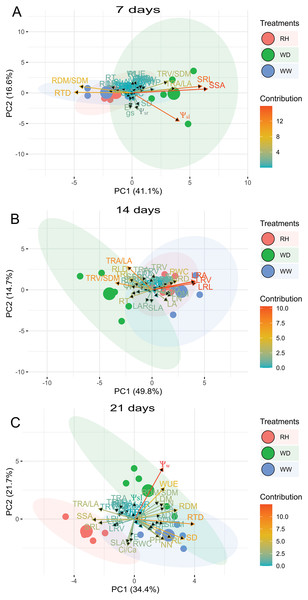
Principal components (PCs) of both C. abyssinica and R. sativus were performed for 7, 14 and 21 days after treatments imposition. Based on scaled values, the first two PCs for C. abyssinica explained 57.7%, 64.5%, and 56.1% of the total variation for 7, 14 and 21 days after treatments imposition, respectively (Fig. 6). At 7 days, PC1 and PC2 had a higher contribution for SSA, Ψsleaf, SRL, RTD, and RDM/SDM. At 14 days, both PC1 and PC2 had a higher correlation with large roots, TRA/LA, TRV/SDM, and RWC; and at 21 days with Ψw, RTD, SD, WUE, RDM and RL (Fig. 6).
Figure 6: Principal Component Analysis (PCA) of Crambe abyssinica.
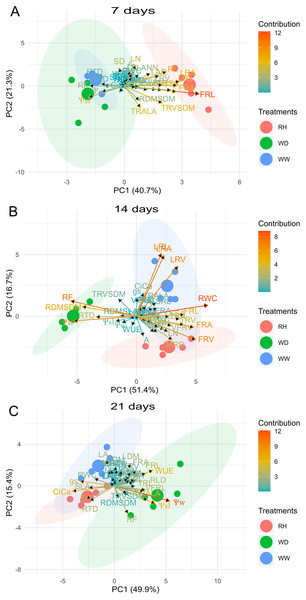
Biplot between PC1 and PC2 showing the contribution of 43 morphological, water relation and gas exchange traits in variability and the segregation of Crambe abyssinica plants under well-watered (WW), water deficit (WD) and after rehydration (RH) condition, at 7 (A), 14 (B), and 21 (C) days.For R. sativus, the first two PCs explained 62.0%, 68.1%, and 65.3% of the total variation for 7, 14, and 21 days after treatments imposition (Fig. 7). At 7 days, fine root length and fine root area, TRV/SDM, LRA and RL contributed in greater proportion to PC1 and PC2 (Fig. 7). At 14 days, RWC, root thickness, large roots and fine root volume large provided the mains contributions; and at 21 days, PC1 and PC2 had a strong correlation with Ψw, Ψsleaf, Ci/Ca, WUE TRA/LA, gS and FRL (Fig. 7).
Figure 7: Principal Component Analysis (PCA) of Raphanus sativus.
Biplot between PC1 and PC2 showing the contribution of 43 morphological, water relation and gas exchange traits in variability and the segregation of Raphanus sativus plants under well-watered (WW), water deficit (WD) and after rehydration (RH) condition, at 7 (A), 14 (B), and 21 (C) days.Discussion
The results of this study confirmed the hypothesis that root changes are involved in the responses of tolerance to water deficit and post-stress recovery in C. abyssinica and R. sativus. The results showed that R. sativus has greater tolerance to water deficit and showed faster recovery of physiological characteristics than in C. abyssinica due to the efficiency of its root system. Very fine (R. sativus) vs. fine roots (C. abyssinica) attributes were decisive to determine tolerance levels.
The duration of the water deficit affected the morphological characteristics of C. abyssinica and R. sativus at different levels. The recovery was determined by the adjustment capacity of these species to the stress. During the 7-days of water deficit, the maintenance of the water status of C. abyssinica and R. sativus can be mainly attributed to the formation of thinner and larger roots. Fine roots make up a large part of the root system and increase the surface area for water extraction (McCormack et al., 2015; Wasaya et al., 2018). In C. abyssinica, a higher specific length and volume of fine roots were observed in addition to the increase in surface area after 7 days. These characteristics are associated with greater efficiency in water absorption and are reported as drought tolerance strategies in chickpea (Ramamoorthy et al., 2016) and wheat (Awad et al., 2018) plants. In addition, the investment of C. abyssinica in thinner roots and longer root length associated with the maintenance of higher water content in the leaves suggests adjustment between root length and diameter as a mechanism to increase water uptake (Comas et al., 2013).
A higher TRA/LA in C. abyssinica corroborates with an increase in water uptake. Plants capable of maintaining a balance between water uptake by the root system and water loss through transpiration are more tolerant to water deficit (He et al., 2017; Tardieu, Draye & Javaux, 2017). Furthermore, the maintenance of specific leaf area and leaf area ratio as observed in C. abyssinica indicates that the plant’s performance was not impaired even under water deficits. This response corroborates the maintenance of photosynthesis (Wellstein et al., 2017).
At the same time that the higher TRL/SDM point to roots as the main drain of photoassimilates produced during stress, the reduction in root tissue density demonstrates the rapid expansion of the root system but with a low accumulation of dry matter (Birouste et al., 2014). The root tissue density controls the specific root length and specific surface area, thus increasing water uptake under water deficit (Wasaya et al., 2018). After rehydration, C. abyssinica reestablished physiological functions, which allowed the resumption of plant growth. Similar results were observed by Braga et al. (2021) which reported that C. abyssinica plants maintained the photosynthesis performance under drought by increasing its water conductivity and water use efficiency.
Photosynthesis was limited in C. abyssinica during the 14 and 21-days of water deficit, associated with a reduction in leaf tissue hydration and constant water use efficiency. The maintenance of WUE in this case is associated with greater investment in roots as corroborated by a higher RDM/SDM and fine root length. There were also root morphological characteristics that contribute to tolerance to water deficit through efficiency in water acquisition and use as previously reported by Koevoets et al. (2016). Furthermore, stomatal closure is an important strategy to prevent water loss by plants (Chaves et al., 2016; Yan, Zhong & Shangguan, 2016; Braga et al., 2021). This can also reduce shoot dry matter to minimize water loss (He et al., 2017; da Cruz et al., 2019).
In R. sativus, photosynthesis and growth were not compromised by the 7-day water deficit possibly due to the maintenance of leaf tissue hydration as observed by the higher relative water content—a characteristic of plants tolerant to drought (Zegaoui et al., 2017). With rehydration after water deficit for 7 days, the increase in length, surface area, and volume of fine roots demonstrated greater efficiency in water absorption in R. sativus (Bristiel et al., 2019).
R. sativus maintained photosynthesis despite the reduction in the relative water content of the leaves. This suggests that the tissue water content was sufficient to support the photosynthetic metabolism with the persistence of the water deficit, which was observed by the osmotic adjustment and increase in water use efficiency at 21 DAT. This led to changes in the anatomy of the leaves that are important indicators of the level of tolerance to water deficit (Souza et al., 2018). The specific leaf area and the leaf area ratio characteristics are indicative of drought-tolerant genotypes when maintained during 14 and 21 days of water deficit as observed in chickpea plants ( Wellstein et al., 2017).
The mechanisms used by R. sativus for the maintenance of water status after 14 and 21 days of water deficits were also involved with an increase in dry matter and reductions in leaf area and root diameter. The maintenance of root dry mater seen in R. sativus (WD14 and WD21) and C. abyssinica (WD21) indicates the capacity of these plants to tolerate the stress due to the ability to develop roots and/or maintain them. This response is associated with greater adaptation to drought conditions (Comas et al., 2013; Koevoets et al., 2016). Furthermore, the increase in root tissue density during a 14-day of water deficit concomitant with the reduction in specific surface area and maintenance of the root length in R. sativus, demonstrate the occurrence of longer roots. Interestingly, these features are seen without an increase in volume of explored soil, which may be associated with water conservation as a strategy to tolerate drought (Luke Mccormack et al., 2012). In contrast, the reduction in the root tissue density and the increase in the specific surface area and root length density—together with the maintenance of the root length observed during the water deficit for 21 days in R. sativus—indicate the development of roots with a low metabolic cost, but with a greater volume of soil explored. This shows that water acquisition is a dehydration-prevention strategy (White & Snow, 2012; Bristiel et al., 2019). In this case, the root alterations of R. sativus demonstrated the use of different adaptive strategies in response to the duration of the water deficit.
The ability to fully recover photosynthesis after water deficit can be considered an indication of greater tolerance to drought (Challabathula, Zhang & Bartels, 2018; Zhang et al., 2018). C. abyssinica could not recover photosynthesis after reestablishing water conditions, despite the use of root strategies to increase water acquisition. A similar response was observed in bean plants exposed to prolonged water deficit and subsequent rehydration (Widuri et al., 2018). However, R. sativus showed a higher rate of plant recovery after stress in addition to tolerating the water deficit for 14 and 21 days. The growth was partially recovered in the two species studied.
The similarity between the adaptive mechanisms employed by C. abyssinica and R. sativus to control water use and consequently maintain physiological activity under water deficit, indicate that the characteristics of the roots were the most determinant to differentiate drought tolerance of plants—especially root diameter (Koevoets et al., 2016; Awad et al., 2018). Thus, the formation of fine roots in C. abyssinica and very fine roots in R. sativus constituted key points for the differentiation of physiological performance between the species (McCormack et al., 2015; Ramamoorthy et al., 2016).
Thinner roots are usually associated with greater volume of soil explored by the larger root surface (Carminati et al., 2017; Ahmed, Passioura & Carminati, 2018). In addition, thinner roots have greater permeability, thus leading to increased efficiency in water acquisition and use (McCormack et al., 2015; Wasaya et al., 2018). This favors better adaptation of plants to drought conditions (Pierret et al., 2007; Bristiel et al., 2019) as observed in R. sativus.
Conclusions
Water restriction affected both C. abyssinica and R. sativus plants, causing changes in their water status and reduction in growth and biomass allocation. Both species have root morphological traits that improve the water uptake. In this sense, the main traits involved in the water deficit tolerance of the two species were the smallest root diameter, and the total length of fine roots (C. abyssinica) or very fine roots (R. sativus). The ability of plants to recover from stress was influenced by the duration of the water deficit and was less efficient after 21 days. Thus, the rehydration was not effective for recovering photosynthesis and growth of C. abyssinica. The lack of very fine roots, more efficient in absorbing water and nutrients, contributed to this response. On the other hand, R. sativus maintained very fine roots throughout the water deficit period and showed greater stress tolerance and faster recovery of physiological functions after rehydration.
Supplemental Information
Crambe abyssinica raw data.
Raw morphophysiological data of Crambe abyssinica.
Raphanus sativus raw data.
Raw morphophysiological data of Raphanus sativus.