Antimicrobial properties, cytotoxicity, colour and mechanical behavior of light-cured resin composites containing modified Novaron
- Published
- Accepted
- Received
- Academic Editor
- Andre Fajardo
- Subject Areas
- Biomaterials, Composites, Materials Science (other)
- Keywords
- Silver-supported material, Resin composites, Mechanical behavior, Antimicrobial property, Cytotoxicity
- Copyright
- © 2022 Han et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ Materials Science) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Antimicrobial properties, cytotoxicity, colour and mechanical behavior of light-cured resin composites containing modified Novaron. PeerJ Materials Science 4:e19 https://doi.org/10.7717/peerj-matsci.19
Abstract
Background
The NOVARON, a silver-based antimicrobial agent derived from inorganic ion exchangers developed by Toagosei and registered by FDA, has effectively indicated the antimicrobial power of silver against a variety of microbes. The objective of this study was to investigate the effect of a silver-supported material (Novaron (N)) on the mechanical behaviour, antimicrobial properties, cytotoxicity and colour of light-cured resin composites.
Methods
Silanized aluminum borate whisker (ABWs) (4 wt%) and nano-zirconia (nano-ZrO2) (2 wt%) were mixed with the resin matrix to obtain the control groups; 4 wt% surface-modified Novaron particles were incorporated into the above matrices as the experimental groups. The surface hardness was tested. Furthermore, the antimicrobial abilities evaluated in vitro with Streptococcus mutans (S. mutans), Fusobacterium nucleatum (F. nucleatum) and Candida albicans (C. albicans) using the live/dead, MTT and colony-forming units (CFUs) assay. Furthermore, the effects on fibroblast growth and colour were test in this study.
Results
The data of the Novaron and control groups were analyzed by Student’s t-test. The results showed that the activities of S. mutans, F. nucleatum and C. albicans biofilms on the composites surface were greatly reduced (p < 0.05) and no significant difference was found in the culture medium (p > 0.05). Extracts taken from the cell culture medium of the specimens were used to evaluate cell viability. The composites did not have an adverse effect on fibroblast growth and colour in this study. The results showed that 4 wt% Novaron incorporated into the resin composites could increase the surface hardness (p < 0.05). Therefore, Novaron is a potential antimicrobial agent applying in light-cured and inorganic nanoparticles reinforced dental resin materials.
Introduction
The development of light-cured dental restorative composites has increased rapidly due to their better aesthetic properties, fewer safety concerns, ease of handling, physical properties similar to dentin and reasonably satisfactory clinical results compared with those of metallic dental amalgams (Wang et al., 2019). Dental composites have been reported to be used in more than 95% of all anterior tooth direct restorations and in approximately 50% of all posterior tooth direct restorations (He et al., 2015). Generally, light-cured dental resin composites consist of an organic resin matrix, inorganic fillers, photo-initiators and accelerators. The most commonly used organic matrices are Bis-GMA and TEGDMA (Zhang et al., 2014a). Inorganic fillers such as zirconium dioxide, silicon dioxide and other glass particles are popularly used to improve the mechanical properties of resin composites (Wille et al., 2016). It is well known that there are different surface properties between the matrix and the fillers; the former is hydrophilic and highly polar, while the latter is generally relatively hydrophobic and non-polar. Surface modification with silane coupling agents is commonly used to increase the interfacial interaction between inorganic fillers and the organic resin matrix (Lung et al., 2016).
However, resin composites have some disadvantages, including excessive wear, inadequate strength, technique sensitivity, dimensional shrinkage, poor marginal adaptation, distortion, and lower resistance to caries (Cherchali et al., 2017; Ibrahim et al., 2020). The main reason for failure is still secondary caries, followed by fracture of restoration. Compared to other restorative materials, dental resin composites restorative materials have been reported to accumulate more bacteria or plaque and more easily form dental biofilms (Almousa et al., 2019; Pietrokovski et al., 2016). Among various bacterial species, Streptococcus mutans is considered to play a major role in the formation and development of plaque biofilms (De Paula et al., 2018). However, other oral microorganisms such as Enterococcus faecalis, Candida albicans and Fusobacterium nucleatum also play an important role in the development and progression of this disease (Melo et al., 2018). Therefore, the development of antibacterial restorative filling materials requires a potent antimicrobial agent that acts against a wide range of oral micro-organisms. Recent studies have paid growing attention to resin composites materials with antibacterial properties, combating microbial growth/proliferation and secondary caries to improve the longevity of restorations. The most common method is incorporation of the filler with an inorganic antibacterial agent (Boaro et al., 2019; Münchow et al., 2020). Compared to organic antibacterial materials, inorganic antibacterial agents have better compatibility as well as long-lasting and wide broad-spectrum antibacterial properties (Wang et al., 2017). For the antibacterial properties of dental resin composites, Ag(silver)-based agents are one of the most commonly used inorganic agents, and silver-zeolite, silver-apatite and other silver-supported materials have been reported to achieve good antibacterial effects (Mocanu et al., 2014).
In our previous research (Chen et al., 2017), we had chosen four different inorganic antibacterial agents (titanium dioxide (TiO2), silver-supported titanium dioxide (Ag/TiO2), silver-supported zirconium phosphate (Novaron), and tetrapod-like zinc oxide whiskers (T-ZnOw)) to fabricated antibacterial composites and investigated their antibacterial activities against oral microorganisms. Novaron had the highest antibacterial property. Novaron consists of uniform fine particles with low moisture absorption and good heat-resistant properties and is easy to mix with matrices. The antimicrobial mechanism of Novaron involves either or both of the following steps: silver ions enter into the cell membranes of bacteria and then inhibit the crosslinked action of polysaccharide to lightly destroy the cell membranes of bacteria; silver ions combine with DNA, interfere with the synthesis of DNA and RNA, inhibition the replication and proliferation of DNA and finally results in bacterial death (Yeluri, Holla & Munshi, 2012).
Therefore, the objective of this study was to investigate the antimicrobial properties of the silver-supported material Novaron in dental composites. Following our previous research, ABWs and Nano-ZrO2 were used as fillers to improve the mechanical properties of resin composites (Zhang et al., 2014b). In this study, we applied silanization methods to modify the fillers, aiming to modify the surface of Novaron. Additionally, as previous research discovered that 4% Novaron offered good antibacterial and mechanical properties and lower cytotoxicity in acrylic resin composites (Han et al., 2015). In this study, we added 4% Novaron into light-cured and nanoparticles reinforced resin to fabricate novel composites, and evaluated the antimicrobial properties against three different microorganisms as well as the mechanical and cytotoxic properties in vitro.
Materials and Methods
Materials
A light-cured resin composites system was used as the parent resin system to test the effect of Novaron incorporation. The composites contain two matrices, BIS-GMA (Sigma-Aldrich, St. Louis, MI, USA) and TEGDMA (Sigma-Aldrich, St. Louis, MI, USA); CQ (Sigma-Aldrich, St. Louis, MI, USA), DMAEMA (Sigma-Aldrich, St. Louis, MI, USA), and BHT (Sigma-Aldrich, St. Louis, MI, USA). Two types of filler materials were purchased to reinforce the mechanical properties of the resin composites: Nano-ZrO2 (granularity: 50–90 nm, Tosoh Co., Ltd., Tokyo, Japan) and ABWs (diameter < 1.5 µm, length: 5–30 µm, Shanghai Whisker Composites Co., Ltd., Shanghai, China). The antimicrobial materials were white silver-supported powders: Novaron AG300 (N, average particle size: 0.9 µm, Toagosei Co., Ltd., Tokyo, Japan) in this study. The silane coupling agent (Z-6030), namely, γ-MPS (Dow Chemical Company, Midland, MI, USA), was purchased to silanize the mixed materials. The other chemicals in this study were analytical grade reagents.
Surface modification of the antimicrobial materials
For the surface treatment of the Novaron, 1.5% γ-MPS (Z-6030) and 10 g of acetone were mixed, and the pH value was adjusted to 4.0–5.0 to control the hydrolysis reaction using acetic acid. The solution was stirred continuously for 1 h to pre-hydrolyse the silane using a magnetic stirring apparatus. Meanwhile, the Novaron powders were immersed in distilled water (Novaron/water weight ratio of 1:10) and completely dispersed with an ultrasonic vibration apparatus for 1 h. Then, the pre-hydrolysed silane was slowly added dropwise into the Novaron suspension, and the mixture was agitated for 1 h at 80 °C. After hot agitation, the suspension was cooled stepwise from room temperature to −80 °C and dried in a vacuum freeze dryer for 24 h to obtain the powders.
The surface analyses of unsilanized and silanized Novaron were examined using XPS (AXIS UltraDLD, Kratos, Japan) with an A1 Ka source (1,486.6 eV). The morphology of the powder and the dispersion in composites were investigated using SEM (NOVA NanoSEM230; FEI Company, Netherlands, Europe).
Specimens
The two resin matrices BIS-GMA (24.8875%) and TEGDMA (74.6625%) at a 1:3 weight ratio were mixed, and 0.25% photosensitizer CQ, 0.15% activator DMAEMA and 0.05% polymerization inhibitor BHT were added. The silanization of nano-ZrO2 and ABWs was according to the previous study (Han et al., 2015). The silanized nano-ZrO2 (20 wt%), ABWs (40 wt%) and Novaron (4 wt%) were incorporated into the resin composites and stirred well to form a resin paste. The experimental groups containing Novaron, while the control groups did not. Table 1 lists the different groups of composition prepared in this study.
| Group | Ingredients |
|---|---|
| Control group | BIS-GMA, TEGDMA, CQ, DMAEMA, BHT, Silane coupling agent: nano-ZrO2, ABWs |
| Novaron group | BIS-GMA, TEGDMA, CQ, DMAEMA, BHT, Silane coupling agent: nano-ZrO2, ABWs, Novaron (4 wt%) |
Then, the resin paste was poured into a round mould and light-cured layer-by-layer. Every layer was 0.2 mm, and the curing light (Elipar™ S10 LED, 3M, USA) was used to cure the resin layer in 10 s. As our previous research (Han et al., 2015), the specimens were standardized to dimensions of 10 mm diameter and 2 mm height. All the specimens were mechanically polished to a high gloss using a grinder-polisher (Phoenix Beta; Buehler Ltd., Germany) and wet abrasive paper. To conduct the biofilm experiments and cytotoxicity tests, the specimen surface was polished to a roughness of 0.18 ± 0.03 µm for the antimicrobial and cytotoxicity tests and 0.02 ± 0.005 µm for the Vickers hardness tests. The specimens were sterilized under ultraviolet light for 2 h on each side for the antimicrobial and cytotoxicity tests.
Mechanical properties test
After the specimens were polished to a surface roughness of 0.02 ± 0.005 µm, the Vickers hardness of specimens randomly chosen from each test group was measured with a micro-hardness tester (HX-1000; Shanghai Taiming Optical Instruments Co., Ltd., Shanghai, China). Three specimens were chosen in each group and three points on each specimen were tested by applying a 50 g (0.49 N) load for 10 s. The results were recorded with PC software.
Live/dead assay of different microorganisms
Three strains of microbes, namely, S. mutans (UA159, gram-positive bacterium), C. albicans (76615) and F. nucleatum (AT25586 gram-negative bacterium) (Shanghai Key Laboratory of Stomatology, Shanghai, China), were used in this study. S. mutans and C. albicans were cultivated under aerobic conditions and F. nucleatum was cultivated under anaerobic conditions in 5 mL of BHI (BD, Franklin Lakes, NJ, USA) at 37 °C for 24 h. The methods of bacterium co-culture were as same as our previous research (Han et al., 2015). Then, the bacterium suspension was adjusted to 1.0 × 106 CFUs/mL for further use. Six disc-shaped specimens for each group were placed in a 48-well plate with 500 µL of BHI and 50 µL of inoculated cell suspension in each well. After 24 h inoculation, the discs with biofilm were transferred to a new 48-well plate, and the planktonic cells in the medium were used in the following experiments.
The method of Live/dead assay was as same as our previous research (Liu et al., 2020). The cells in the biofilms on discs were harvested with 1 mL of BHI with mild sonication and pipetting and stained using a live/dead cell viability kit (Molecular Probes, Invitrogen, Eugene, OR, USA) for 15 min in darkness. Live cells were stained with Syto 9 to produce green fluorescence, while dead cell membranes were stained with propidium iodide to produce red fluorescence. Separately, the planktonic cells in the medium were collected and similarly live/dead stained. Each test was performed at n = 6. The stained specimens were examined by CLSM (Leica TCS SP2, Germany).
MTT assay of cell metabolic activity
The MTT assay is a colorimetric assay to estimate the metabolic activity of cells. The 24 h biofilm discs were transferred to a new 48-well plate, and 300 µL of MTT dye (0.5 mg/mL MTT in PBS) was added to each well. Meanwhile, the collected medium with planktonic cells from each well was transferred to a tube containing 30 µL of MTT dye. The specimens of S. mutans and C. albicans were incubated at 37 °C in an aerobic incubator and F. nucleatum in an anaerobic incubator for 4 h. During this process, metabolically active cells reduced the MTT to purple formazan. After 4 h, the discs were transferred to a new 48-well plate, and 300 µL of DMSO (Sigma, Ronkonkoma, NY, USA) was added to solubilize the formazan crystals, while the planktonic cells were collected by centrifugation at 5,000 × g for 4 min, adding 300 µL of DMSO. After incubation for 20 min with gentle mixing at room temperature in the dark, 200 µL of the DMSO solution was transferred to a new 96-well plate. The absorbance at 590 nm (OD590) was recorded via a multiwell microplate reader (Labsystem Multiskan EX, USA). The higher absorbance indicated a higher formazan concentration, which indicated a higher metabolic activity of the cells. Six replicates were tested for each group (n = 6).
Colony forming unit counts (CFUs)
Two types of agar plates were prepared in this part: the BHI agar plates were for S. mutans and C. albicans, and the BHI blood agar plates were used to culture F. nucleatum. The cells in the 24 h biofilms on discs were rinsed via mild sonication and were pipetted. Then, the cell suspensions were serially diluted to 10−6, and 50 µL of the diluted cell liquid was spread onto a BHI agar plate or BHI blood agar plate and cultured at 37 °C for 48 h for CFUs analysis (n = 6). Separately, the CFUs of the planktonic cells from each well were also measured.
Cytotoxicity of the composites eluent
For the cell cytotoxicity test, eluent solutions of specimens were prepared according to ISO 10993-5 and ISO 10993-12. Sterile specimens were immersed in DMEM and agitated for 24 ± 2 h at 37 °C to obtain the extracts from the specimens. The surface/volume ratio of the specimen and the medium was 1.25 cm2/mL. After incubation, the extracts were filtered by 0.22 µm filters into sterile tubes and diluted two-fold with fresh DMEM for testing. The negative control groups were DMEM without the eluent solution.
Gingival fibroblasts cultured in DMEM supplemented with 10% fetal calf serum with 100 U/mL penicillin and 100 mg/mL streptomycin (Gibco BRL, New York, NY, USA) at 37 °C in an air atmosphere containing 5% CO2 at 100% relative humidity. A seeding density of 4,000 cells/well was used in 96-well plates, with 200 µL per well. After 24 h incubation at 37 °C with 5% CO2 in air, the culture medium was removed and replaced with equal volumes of the eluent solution and a 2-fold dilution. Meanwhile, the negative groups were treated with DMEM. The cells were cultured for another 24 h, and then, 20 µL of sterile-filtered MTT was added to each well. After incubation in a darkroom for 4 h at 37 °C, the unreacted dye was removed, and 200 µL/well of DMSO was added. The plates were then slightly stirred at room temperature for 10 min, and the solution absorbance was measured via a microplate reader (Labsystem Multiskan EX, USA) at 490 nm. The absorbance of the negative groups was set as 100%. The fibroblast viability for cells cultured with eluents = absorbance with eluents/absorbance of negative control.
Colour change measurement
To evaluate the colour changes, specimens measuring 10 mm in diameter and 2 mm in thickness were prepared. Six samples were prepared from each material. After preparation, the samples were polished to a surface roughness of 0.02 ± 0.005 µm and immersed in stilled water in darkness at 55 ± 2 °C for approximately 30 min. A spectrophotometer (Color i7; X-Rite, Grand Rapids, MI, USA) was used to record the CIE L*a*b* parameters with a D65 illuminant on a white ceramic tile. The CIEL ab system is composed of three respective axes: L* is the lightness from 0 (black) to 100 (white), a* represents the red (+a* value)—green (−a* value) axis, and b* represents the blue (−b* value)—yellow (+b* value) axis. The colour change (ΔE*) was calculated according to the following Eq. (1) (Tsubone et al., 2012):
(1) where L*, Δa*, and Δb* represent the difference values of L*, a*, and b* between the Novaron groups and the control groups, respectively. ΔE < 1.0 indicated that the change in colour could not be detected; 1.0 < ΔE < 3.3 indicated that the change in colour could not be distinguished; and ΔE > 3.3 showed that the difference in colour was obvious.
Statistical analysis
The normal distribution and homogeneity of all the data were checked using the Shapiro-Wilk test. Then, the data of the Novaron and control groups were analysed by Student’s t-test using SPSS 19.0 statistical software at a significance level of p < 0.05.
Results
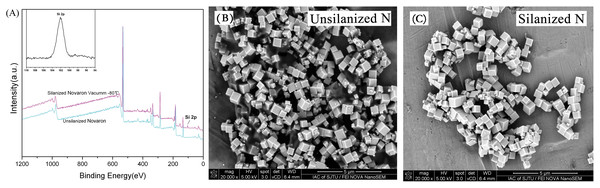
Figure 1A shows the XPS data of the Novaron surface before and after silanization. The band at approximately 102 eV in the spectrum of the Si-2p groups observed was attributed to silanized Novaron. Figures 1B and 1C present the SEM images of the unsilanized and silanized Novaron samples, respectively. These images showed good dispersibility of the fillers after surface treatment, and this property could increase the interfacial contact between fillers and the matrix.
Figure 1: Surface silanization.
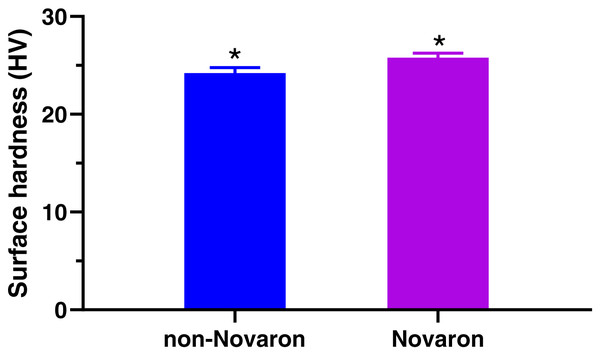
(A) XPS spectrum of Novaron and the Si-2p groups at approximately 102 eV after surface treatment. (B & C) Representative SEM images of Novaron before and after silanization, showing good dispersibility after surface treatment.Figure 2 presents the surface hardness results. Statistical analysis revealed that the surface hardness was enhanced significantly with the addition of Novaron compared to that of the control groups (p < 0.05).
Figure 2: Surface hardness.
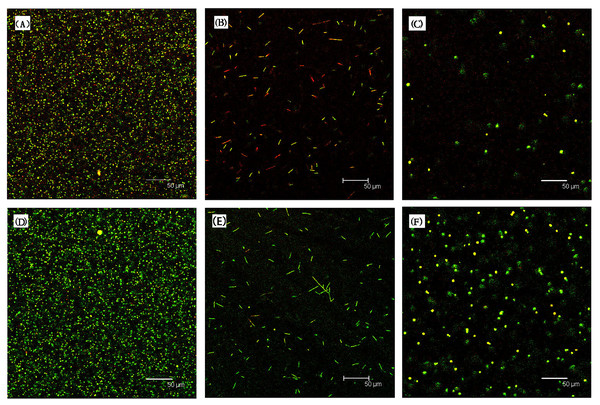
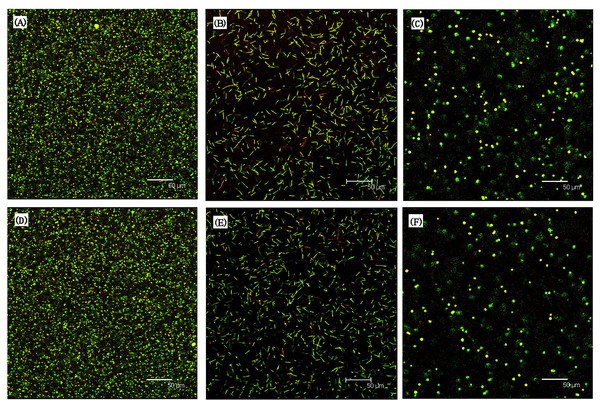
The results indicated that the surface hardness were significantly enhanced upon the addition of 4 wt% Novaron groups (p < 0.05). The asterisks means that there were significant differences between control groups and the Novaron groups.Figure 3 shows the live/dead images are for biofilms on resin discs; the left columns represent the control groups with different cells, while the right columns represent the Novaron groups. For S. mutans (A), F. nucleatum (B) and C. albicans (C), the resin composites containing Novaron had more compromised cells than the control groups did (D, E, F). Figure 4 shows the live/dead results of planktonic cells in the medium. There were no obvious differences between the Novaron and control groups for any of the different microbes. These results indicate that the Novaron-containing resin inhibited cell growth on its surface, but the cells distant from its surface were still primarily alive.
Figure 3: Represented live/dead images for biofilms on resin disks.
For S. mutans (A), F. nucleatum (B) and C. albicans (C), the resin composites containing Novaron had more compromised bacteria compared to control groups (D–F).Figure 4: Showed the live/dead results of planktonic bacteria in the medium.
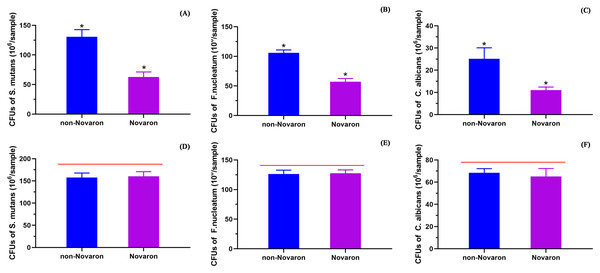
S. mutans (A), F. nucleatum (B) and C. albicans (C) were the resin composites containing Novaron and (D–F) were the control groups. There were no obvious different between the Novaron groups and control groups of all different bacteria.Figure 5A (S. mutans), B (F. nucleatum) and C (C. albicans) present the MTT results for the biofilms on the resin surfaces of the Novaron groups, and Figs. 5D–5F are the results for the planktonic cells in the culture medium that were distant from the resin surface. The results showed that the Novaron groups achieved a significantly lower MTT absorbance than that observed in the control groups (p < 0.05), while there were no significant differences between the control and Novaron groups in terms of planktonic cells in the culture medium (p > 0.05).
Figure 5: The MTT results.
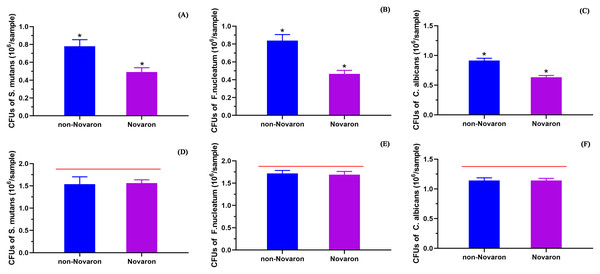
(A) (S. mutans), (B) (F. nucleatum) and (C) (C. albicans) presented the results of biofilms on the resin surface and (D–F) were for the planktonic bacteria in culture medium away from the resin surface. The results showed Novaron groups achieved significantly lower MTT absorbance than the control groups (p < 0.05) while there were no significant differences between control groups and the Novaron groups of planktonic bacteria in culture medium (p > 0.05). The asterisks means that there were significant differences between control groups and the Novaron groups, and the red lines means there were no significant differences between the control groups and the Novaron groups.Figure 6A (S. mutans), B (F. nucleatum) and C (C. albicans) present the CFU results on the Novaron groups resin surface, and the results for the planktonic cells in the culture medium are presented in Figs. 6D–6F. The results were the same as the MTT results. The Novaron groups achieved significantly lower CFUs than the control groups did (p < 0.05), while there were no significant differences between the control groups and Novaron groups planktonic cells in the culture medium (p > 0.05).
Figure 6: CFUs results.
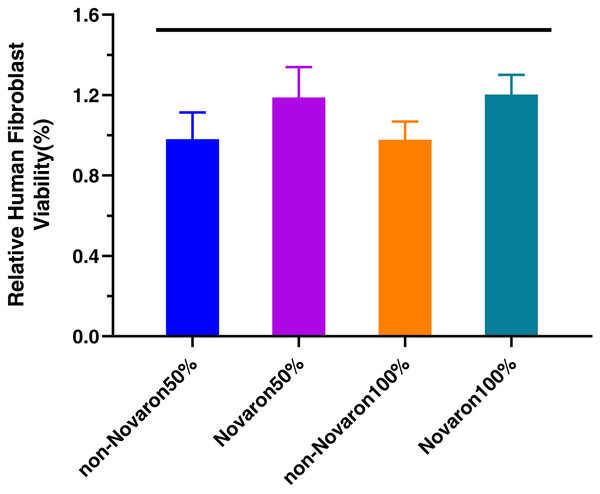
(A) (S. mutans), (B) (C. albicans) and (C) (F. nucleatum) were the results of biofilms on the resin surface and planktonic bacteria in culture medium (D–F). Novaron groups achieved significantly lower CFUs than the control groups (p < 0.05) while there were no significant differences between control groups and the Novaron groups of planktonic bacteria in culture medium (p > 0.05). The asterisks means that there were significant differences between control groups and the Novaron groups, and the red lines means there were no significant differences between control groups and the Novaron groups.Figure 7 shows the results regarding the fibroblast cytotoxicity of resin composites, indicating that there were no significant differences in relative cell viability between the undiluted extracts and two-fold diluted eluents (p > 0.05).
Figure 7: Human fibroblast cytotoxicity.
The results indicated that there were no significant differences of relative cell viability between the undiluted extracts and two-fold diluted eluents (p > 0.05). The red lines means there were no significant differences between the control groups and the Novaron groups.The results of the initial colour measurements of the L*, a* and b* axes are presented in Table 2. The colour change of the chromaticity of the specimen after the addition of 4% Novaron is shown in Table 3. The ΔE values were less than 1.0, indicating that humans could not detect the differences between these two groups.
| Groups | L* | a* | b* |
|---|---|---|---|
| Control groups | 88.240 ± 0.493 | −0.763 ± 0.061 | 1.125 ± 0.172 |
| Novaron groups | 89.273 ± 0.456 | −0.808 ± 0.082 | 1.103 ± 0.266 |
| ΔL* | Δa* | Δb* | ΔE | |
|---|---|---|---|---|
| Novaron groups | 1.033 | 0.045 | 0.022 | 0.535 |
Discussion
Uncoated inorganic materials, especially nanoparticles, normally have a tendency to agglomerate. The effect of aggregation of the materials is hindered by combining them into the resin composites (Chouirfa et al., 2019). Surface modification with silane coupling agents has been one way to stabilize the fillers and disperse the fillers into uniform resin composites (Zane et al., 2016). Because of the different surface properties between the matrix and the fillers, the interphase quality between them plays a major role in the ultimate properties of the composites materials (Jiao et al., 2014). There are several methods available to quantify the interfacial adhesion of fillers in composites materials. Surface modification with silane coupling agents is commonly used to improve interfacial adhesion in filler-reinforced resin composites (Aydinoglu & Yoruc, 2017). The most common silane used in dental composites is γ-MPS, which is a bi-functional monomer, with hydroxymethyl groups substituted by hydroxyl groups attaching to the fillers (Han et al., 2015; Liu et al., 2018). γ-MPS also contains C=C bonds, which react with the resin matrices during the curing process. Therefore, the silane coupling agent γ-MPS established chemical bonds between the fillers and the resin composites as a bridge. Surface modification with γ-MPS resulted in the observed Si absorption peak, indicating that the silane coupling agents were successfully grafted onto the filler surface (Han et al., 2015; Matinlinna, Lung & Tsoi, 2018). After silanization by the vacuum freeze-drying method, the morphology of the fillers was evaluated. The images of Fig. 1 showed good dispersibility of the fillers after surface treatment after silanization.
Based on previous research, ABWs and Nano-ZrO2 were selected as reinforced filler materials incorporated into the resin matrix. Aluminium borate whiskers with a single crystal structure have been successfully used as a reinforcement for metal or resin matrix composites. Nano-ZrO2 containing nanoparticles exhibit the best anti-wear properties, and zirconium dioxide possesses excellent properties such as high hardness, strength and fracture toughness as well as outstanding wear and chemical corrosion resistance performance (Han et al., 2015). Novaron exhibited uniform fine particles with low moisture absorption capability and good heat-resistant properties. This material can be easily mixed into matrices. In addition, it has high physical and chemical stability along with superior discoloration resistance during processing or use (Yeluri, Holla & Munshi, 2012).
In the present study, S. mutans, C. albicans and F. nucleatum were used to examine the antimicrobial activities of Novaron in a resin matrix with direct contact and the planktonic cell test. Streptococcus mutans is a gram-positive bacterium and represents one of the main species in cariogenic biofilms responsible for secondary caries (Florez et al., 2016). Oral C. albicans has been recognized as one of the contributing factors of denture stomatitis. Biofilm formation on dental materials and the subsequent colonization of microbial cells may cause secondary caries and gingivitis (Oktay et al., 2019). F. nucleatum is a gram-negative anaerobe correlated with increased probing depth and progressive periodontal ligament reduction in periodontitis. Periodontitis enhances the loss of tooth attachment and the development of root caries and leads to the failure of Class V restorations (Wang et al., 2016). The biofilm composition may influence the outcome of caries treatments and the killing efficacy of antibacterial agents. Therefore, new antimicrobial restorative materials should be tested against multispecies biofilms.
Novaron is a silver-supported inorganic agent. Silver is well known for its broad-spectrum antimicrobial properties and good biocompatibility with human cells; thus, it has been widely used in medical and other fields. Silver is antimicrobial against a wide range of microorganisms: bacteria, fungi and certain viruses, including antibiotic-resistant strains (Cao et al., 2017). Silver ions, as released antimicrobial agents, have been incorporated into composites mixtures in an attempt to achieve significant antimicrobial performance. However, as these silver ions are released, the generation of voids can negatively affect the mechanical properties of the composites. Burst release is another concern of this technique (Chatzistavrou et al., 2015). Conversely, non-released agents, such as silver-supported fillers, are known to maintain remarkable mechanical properties after ageing since the antimicrobial component is not released over time. Novaron is a silver-supported inorganic antibacterial agent and has excellent antimicrobial efficacy against a wide range of microorganisms (Cao et al., 2018). The antimicrobial mechanism of Novaron is presumed to involve either or both of the following steps: silver ions form metal-organic complexes with sulfhydryl groups in the cell walls of bacteria and fungi, generally inactivating essential enzymes responsible for energy metabolism (Dias et al., 2019). Silver ions also activate oxygen, which is converted into oxygen free radicals by the action of light energy in air or water as a result of the catalytic action of silver, attacking the respiratory chain and cell division, leading to cell death (Xu et al., 2016). Therefore, a further study should investigate the antimicrobial activity over a long period. We chose different methods (Figs. 3–6) to test the composites antimicrobial ability of biofilms and planktonic bacteria and yeast. These results indicated that the Novaron-containing resin inhibited cell growth on its surface, but the cells distant from its surface were still primarily alive. Previous studies suggested that there was no release of silver ions for a long time (Yoshida, Tanagawa & Atsuta, 1999), which was in agreement with the results of this study. The antibacterial activity of the composites incorporating Novaron can possibly last for long periods because the composites inhibit the growth of cells not by releasing the silver ions from the composites but through their direct contact with the bacteria (Kuroki et al., 2010).
It is equally important for new antibacterial composites to be non-cytotoxic and have good biocompatibility. Many studies have reported similar results that Ag+ is nontoxic to the human body at a low concentration (Liu & Man, 2017; Lu et al., 2017; Natarajan et al., 2016). Cells from various organs or tissues usually display differential susceptibility. Gingival fibroblasts are accessible if the composites are to be applied in the clinic (Ren et al., 2019). To exclude the effect of resin monomers on the cytotoxicity assay, the resin discs were cleaned by ultrasound, dried and left undisturbed for 24 h before sterilization. Typical saliva flow is approximately 1,000–1,500 mL/day for an average person. Hence, diluting the original extract 128-fold yields a total of 1,280 mL of culture medium, which can be used to approximate the amount of saliva in the mouth over 24 h (Zhang et al., 2013). The present study in Fig. 7 demonstrated that the relative cell viability percentages of the two-fold diluted and undiluted eluent groups were all greater than 90% after 24 h incubation and were classified as non-cytotoxic and slightly cytotoxic, respectively. In the present study, even at the two-fold dilution, with a total solution volume of approximately 1/2 of the saliva volume per day in vivo, the Novaron groups still exhibited nearly 100% fibroblast viability (Han et al., 2015). According to ISO 10993-5:2009, the resin composites could achieve potential antimicrobial activities without compromising fibroblast cytotoxicity, the material was qualified.
Broad optical properties depending on the nanoparticle diameter, refractive index near the nanoparticle surface, and aggregation are also beneficial features of this material (Stencel et al., 2018). Previous investigations have suggested some thresholds of perceptible color difference. ΔE values lower than approximately 3.3 are acceptable (Vichi, Ferrari & Davidson, 2004). The high surface area to mass ratio of Novaron allows better antimicrobial activity at a lower concentration without significantly compromising the composites colour.
Conclusions
The present study investigated the effects of Novaron addition in nano-ZrO2/ABW resin composites on the mechanical activity, antimicrobial properties, cytotoxicity and colour. The results showed that 4 wt% Novaron incorporated into the resin composites could increase the surface hardness. Antimicrobial functions were obtained without compromising the biocompatibility or colour. Therefore, 4 wt% Novaron may have wide applicability in other composites, bonding systems, sealants and cements. These novel antimicrobial resin composites may be promising for inhibiting oral biofilms and secondary caries.