Preparation, Characterization and Electron Paramagnetic Resonance (EPR) Spectroscopic Studies of OXANOH
DOI:
https://doi.org/10.5530/fra.2018.2.11Keywords:
Ischemia-reperfusion injury, Reactive oxygen species, Perfusion, Lung damage, Scanning electron microscopyAbstract
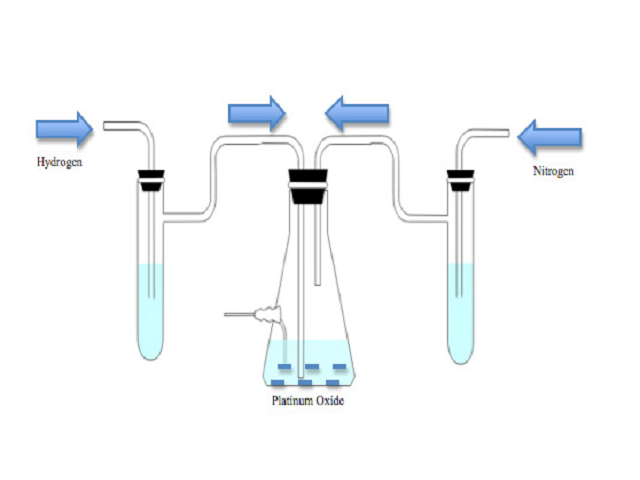
Objective: Hydrogenation of the stable free radical, 2-Ethyl-2,5,5-trimethyl-3-oxazolidinoxyl (OXANO•), converts it to the intracellular spin trap, 2-Ethyl-1-hydroxy-2,5,5-trimethyl-3-oxazolidine (OXANOH). Methods: Due to the flammable nature of the reaction, this procedure must be carried out with extreme care. OXANO• (10 mM) was diluted in 50 ml of Krebs-Henseleit buffer (pH 7.4) and hydrogenated in a nitrogen atmosphere in the presence of Platinum (IV) Oxide Hydrate (100 mg). Results: The reaction was completed in approximately 45 min, and was confirmed by the disappearance of the three-electron paramagnetic resonance (EPR) signals (1:1:1). Additionally, the absorption spectra were measured for both OXANOH and OXANO• in the ultraviolet (UV) and visible light regions. Conclusion: OXANOH is an extremely valuable oxygen free radical spin trap due to its ability to trap both extra and intracellular free radicals in biological tissues.
Downloads
Metrics