Abstract
In 1919, Houssay and Negrete reported that venoms of Theraphosidae spiders induced neuromuscular blockade. In 1993, a purified toxin from Grammostola spider venom was found to block the P-type voltage-dependent calcium channel (VDCC), causing neuromuscular blockade. We studied the mode of action of Theraphosa blondii venom, a large Theraphosidae spider from Northern Brazil, Venezuela, and The Guyanas in mouse phrenic nerve-diaphragm preparation. This venom elicited a partially reversible neuromuscular blockade and did not depress directly evoked twitches or alter the membrane potential. Neostigmine produced only a poor antagonistic effect on partially blocked diaphragms. However, completely blocked miniature endplate potentials (m.e.p.ps) were reverted by neostigmine. These results can be explained by the presence of toxins in the venom that interact with the endplate receptor at the acetylcholine sites (curaremimetic toxins) and toxins that inhibit the P-type voltage-dependent calcium channel (VDCC) (omega -toxins). This study shows that Theraphosidae venoms, especially those of the Theraphosa blondii, are a source of curaremimetic toxins and omega -toxins of possible interest as tools in bioscientific research.
spider venom; mouse diaphragm preparation; neuromuscular junction; Theraphosa blondii
Short Communication
Neuromuscular blocking action of the Theraphosa blondii spider venom
M. D. Fontana1 CORRESPONDENCE TO:
M. D. Fontana - Departmento de Farmacologia, Faculdade de Ciências Médicas, Universidade Estadual de Campinas, UNICAMP, Caixa Postal 6111, 13083-970, Campinas, SP, Brazil
Phone: 55-19-788-7185/8173. Fax : 55-19-289-2968.
fontana@obelix.unicamp.br
, H. S. M. Lucas2, O. Vital Brazil1
CORRESPONDENCE TO:
M. D. Fontana - Departmento de Farmacologia, Faculdade de Ciências Médicas, Universidade Estadual de Campinas, UNICAMP, Caixa Postal 6111, 13083-970, Campinas, SP, Brazil
Phone: 55-19-788-7185/8173. Fax : 55-19-289-2968.
fontana@obelix.unicamp.br
, H. S. M. Lucas2, O. Vital Brazil1
1 Department of Pharmacology, School of Medical Sciences, State University of Campinas, UNICAMP, Brazil, 2 Laboratory of Venomous Arthropods, Butantan Institute, São Paulo, São Paulo State, Brazil.
ABSTRACT: In 1919, Houssay and Negrete reported that venoms of Theraphosidae spiders induced neuromuscular blockade. In 1993, a purified toxin from Grammostola spider venom was found to block the P-type voltage-dependent calcium channel (VDCC), causing neuromuscular blockade. We studied the mode of action of Theraphosa blondii venom, a large Theraphosidae spider from Northern Brazil, Venezuela, and The Guyanas in mouse phrenic nerve-diaphragm preparation. This venom elicited a partially reversible neuromuscular blockade and did not depress directly evoked twitches or alter the membrane potential. Neostigmine produced only a poor antagonistic effect on partially blocked diaphragms. However, completely blocked miniature endplate potentials (m.e.p.ps) were reverted by neostigmine. These results can be explained by the presence of toxins in the venom that interact with the endplate receptor at the acetylcholine sites (curaremimetic toxins) and toxins that inhibit the P-type voltage-dependent calcium channel (VDCC) (w -toxins). This study shows that Theraphosidae venoms, especially those of the Theraphosa blondii, are a source of curaremimetic toxins and w -toxins of possible interest as tools in bioscientific research.
KEY WORDS: spider venom, mouse diaphragm preparation, neuromuscular junction, Theraphosa blondii.
INTRODUCTION
Brazil and Vellard (2) and Vellard (6) reported that venoms of Theraphosidae spiders induce a short period of agitation, flaccid paralysis, and death by respiratory failure in animals. Houssay and Negrete (4) demonstrated that the venom of these spiders causes blockade of neuromuscular transmission. Lampe (5) purified a peptide toxin from the venom of a Theraphosidae spider (Grammostola spatulata) that blocks the P-and N-type voltage-dependent calcium channels (VDCC). VDCC blockade is, therefore, the mechanism or one of the mechanisms by which Grammostola spider venom, of the Theraphosidae family, induces neuromuscular blockade. In this work, we studied the mechanism of venom action from Theraphosa blondii, a very large spider from the Amazon valley in Brazil, Venezuela, and The Guyanas, which belongs to another group of Theraphosidae that includes the most venomous Theraphosidae spiders (6). The result of this study indicates the presence of toxins in this venom that interact with the endplate receptor at the acetylcholine sites (curamimetic or a -toxins), suggesting VDCC-blocking toxins (w -toxins) occur in the venom.
Venom was extracted in the Laboratory of Venomous Arthropods at the Butantan Institute from specimens captured in Tucurui, Pará State, and Balbina, Amazonas State, by electrical stimulation according to Barrio and Vital Brazil (1). It showed LD50 of 1.8 (1.6-2.1 mg/kg) in white mice, producing flaccid paralysis. The action of T. blondii venom on neuromuscular transmission was studied in mouse phrenic nerve-diaphragm preparation. Muscle contractions were recorded on a Gould model 30 VT404 physiograph, by a Gould universal amplifier, model 134615-58. Phrenic nerve-diaphragms were isolated from male Swiss mice (30-40g) and suspended in a glass organ bath, containing 5-10 mL of Tyrode solution (composition in mM: NaCl, 136.8; KCl, 2.7; CaCl2, 1.8; NaHCO3, 11.9; MgCl2, 0.25; NaH2PO4, 0.3; and glucose, 11.0) at 37° C and oxygenated with 95% O2-5% CO2. The phrenic nerve was stimulated with supramaximal rectangular pulses of 0.2 ms duration at a frequency of 0.1 Hz. Rectangular pulses of 50-100 V, 2.0 ms duration and 0.1 Hz were used for direct muscle stimulation of the diaphragm.
Bioelectrical potentials were measured with the diaphragms mounted horizontally in 3 mL perspex organ baths. The bath fluid, temperature, and oxygenation were as described above. Intracellular bioelectrical potentials were recorded in the endplate region, using conventional glass microelectrodes filled with 3M KCl (resistance 5 20 MW ). Transmembrane potentials of five fibers in each preparation were measured using a Bimos operational amplifier (model CA3140) with a typical input impedance of 1.5 TW . Oscilloscope-stored records of m.e.p.ps were photographed before and at various times after venom addition to the bath. The results are expressed as means ± S.E.M. of five experiments or observations. The significance of differences between means was determined using Students t test with p<0.05 significance level.
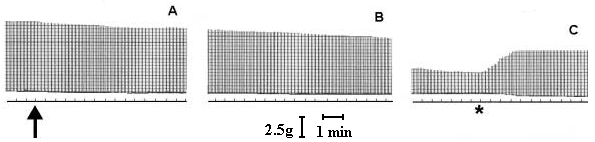
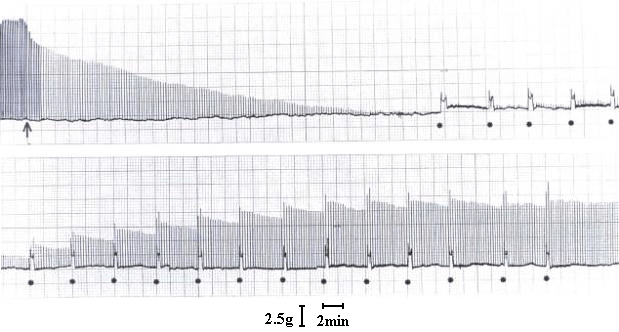
At a concentration of 7.5 µg/ml, T. blondii venom elicited complete neuromuscular blockade (30± 2.5 min) in mouse phrenic nerve-diaphragm preparation (n=5). This blockade was poorly antagonized by neostigmine (neostigmine methyl sulfate, 9.0 mM, Figure 1) and partially reverted by washing the preparation (Figure 2). In the curarized (14.6 µM d-tubocurarine) preparation under direct stimulation, venom did not depress the twitches (Figure 3). The resting membrane potential of muscle fibers was not altered by the venom. T. blondii venom (7.5 µg/mL) reduced the amplitude of the m.e.p.ps and abolished them (Figure 4 B and C) after a latency of 30± 5 min (n=5). Neostigmine (9.0 mM) (Roche, Rio de Janeiro) reversed the blockade (Figure 4D). Frequency of m.e.p.ps before venom and after neostigmine was nearly the same (Figure 4 A and D). 4-Amynopiridine (0.213 mM) (Sigma, Chemical Co, St. Louis, MO) did not antagonize the m.e.p.p. venom-induced blockade.
Effect of T. blondii venom on mouse phrenic nerve-diaphragm preparation. The preparation was indirectly stimulated using supramaximal voltage at a frequency of 0.1 Hz and pulse duration of 0.2 ms. In A, twitch tension before and at the arrow venom addition (7.5 µg/ml). In B, 15 min after venom addition. In C, 30 min after venom addition the partial neuromuscular block, and (*) neostigmine (3 µg/ml) addition.
Effect of T. blondii venom on mouse phrenic nerve-diaphragm preparation. At arrow, the preparation was indirectly stimulated using supramaximal voltage at frequency of 0.1 Hz and pulse duration of 0.2 ms. Venom addition (7.5 µg/ml) at arrow, and (·) indicates preparation washing at 5-min interval when neuromuscular block is present.
The preparation was indirectly stimulated using supramaximal voltage at a frequency of 0.1 Hz and pulse duration of 02 ms. At arrow, the preparation was treated with D-tubocurarine (D-tc, 14.6 µM). The (*) indicates that directly stimulation was carried out at 80 V using 2.0 ms pulses at a frequency of 0.1 Hz and T. blondii venom was added to the bath.
Action of T. blondii venom on the miniature EPPs of mouse phrenic nerve-diaphragm. The m.e.p.ps were recorded as described in the text. A = before venom (control). B and C = 15 and 30 min, respectively, after venom addition (7.5 µg/ml). D = 10 min after neostigmine (9.0 mM) addition.
Decrease in the miniature EPP amplitudes induced by T. blondii venoms and neostigmine antagonistic effect on the blockade of these potentials demonstrated that at least one of the venom toxins interacts reversibly with the endplate nicotinic receptor at the acetylcholine (ACh) sites (curarimetic actions). The lack of 4-aminopyridine effect on m.e.p.p. blockade excludes the presence of a toxin in the venom that induces endplate nicotinic receptor desensitization, since aminopiridines are known to inhibit desensitization of the endplate nicotinic receptor (7-9). Partial reversibility of neuromuscular blockade induced by T. blondii venom and the poor antagonistic effect of neostigmine on a venom-induced partial blockade can be explained by supposing that toxins are present in the venom that either interact irreversibly with the endplate nicotinic receptor or inhibit ACh release by nerve impulses. This latter supposition is favored by the isolation of a toxin from Grammostola spatulata, w-Grammotoxin SAI, which blocks VDCC. About the same m.e.p.p frequency before blockade by the venom and after restoration by neostigmine also confirms this supposition. It may be inferred that T. blondii venom contains curaremimetic toxin(s) and toxin or toxins similar to those found in venoms of the funnel web spider, Agelenopsis aperta, and the Theraphosidae, Grammostola spatulata, which inhibit P-type VDCC. This study indicates that venoms from the Theraphose group (Acanthoscurria, Phormictopus, Lasiodora, Theraphosa) of the Theraphosidae family may be a source of curarimimetic and VDCC blocker toxins [a and w-toxins according to the Cruz et al. nomenclature (3)] of possible importance as tools in bioscientific research.
ACKNOWLEDGEMENTS
This work was supported by CNPq (Brazil). We thank Dr. Stephen Hyslop for the English editing.
Received July 27, 2001
Accepted June 6, 2001
-
1BARRIO A., VITAL BRAZIL O. Ein neues Verfahren der Giftenhme lei Spinnen. Experienti, Basel, 1950, 6, 112.
-
2BRAZIL V., VELLARD J. Contribuição ao estudo do veneno das aranhas: 2a Memória. Mem. Inst. Butantan, 1926, 2, 243-99.
-
3GRAY WR., OLIVEIRA B. Peptide toxins from venomous Conus snails. Ann. Rev. Biochem, 1988, 57, 665-700.
-
4HOUSSAY BA., NEGRETE J. Nuevos estudios experimentales sobre la acción fisiológica de las ponzoñas delas arañas. Rev. Inst. Bact. Depart. Nac. Hig., 1919, 2, 189.
-
5LAMPE BA. Isolation and pharmacological characterizations of w -Grammotoxin SAI, a novel peptide inhibitor of neuronal voltage-sensitive calcium channel responses. Mol. Pharmacol, 1993, 44, 451-60.
-
6VELLARD, J. Le Venins des Araignées Paris: Masson, 1936. 312p.
-
7VITAL BRAZIL O., FONTANA MD., PAVANI NJP. Effect of 4-aminopyridine on end- plate receptor desensitization caused by carbachol. Eur. J. Pharmacol, 1983, 86, 199-205.
-
8VITAL BRAZIL O., FONTANA MD., HELUANY NFS., LAURE CJ. Mode of action of the coral snake Micrurus spixii venom. J. Nat. Toxins, 1995 , 4, 19-33.
-
9VITAL BRAZIL O., FONTANA MD., HELUANY NF. Nature of the postsynaptic action of crotoxin at the guinea pig diaphragm. J. Nat. Toxins, 2000, 9, 33-41.
Publication Dates
-
Publication in this collection
16 Sept 2002 -
Date of issue
2002
History
-
Accepted
06 June 2001 -
Received
27 July 2001