Abstract
Pharmacological substances such as adenosine deaminase (ADA), collagen, histamine, IgE, myoglobin, and nerve growth factor (NGF) are endogenously present in animals. Research from this laboratory reported decreased levels of ADA, histamine, IgE, and NGF in organs of mice injected with sub-lethal doses of cobra venom. The goal of this research is to observe the levels of ADA, collagen, histamine, IgE, myoglobin, and NGF in certain organs of mice injected with venom from the bee Apis mellifera. Adult Balb/c female mice IM injected with half lethal dose of bee venom were sacrificed after 2 and 8 hours for removal of organs. The homogenates of the organs were assayed by ELISA for ADA, collagen, histamine, IgE, myoglobin, and NGF using respective antisera. Organs from mice injected with PBS were used as controls. It was observed that there were decreased levels of ADA, collagen, histamine, IgE, myoglobin, and NGF in certain organs after 2 h and tremendous decrease after 8 h. This is the first report showing the pharmacokinetics of ADA, collagen, histamine, IgE, myoglobin, and NGF as consequence of honeybee envenomation.
honeybee venom; adenosine deaminase; collagen; histamine; IgE; myoglobin; nerve growth factor
Original paper
SUB-LETHAL INJECTION OF HONEYBEE VENOM DECREASED THE LEVELS OF ENDOGENOUSLY PRESENT SUBSTANCES IN ORGANS OF MICE
B. V. LIPPS1 CORRESPONDENCE TO:
B. V. LIPPS - Ophidia Products Inc., 11320 South Post Oak, Suite 203, Houston, Texas 77035 USA.
Fax: 1 713 663-7290
bvl@ophidia.com
CORRESPONDENCE TO:
B. V. LIPPS - Ophidia Products Inc., 11320 South Post Oak, Suite 203, Houston, Texas 77035 USA.
Fax: 1 713 663-7290
bvl@ophidia.com
1 Ophidia Products Inc., 11320 South Post Oak, Suite 203, Houston, Texas 77035 USA.
ABSTRACT: Pharmacological substances such as adenosine deaminase (ADA), collagen, histamine, IgE, myoglobin, and nerve growth factor (NGF) are endogenously present in animals. Research from this laboratory reported decreased levels of ADA, histamine, IgE, and NGF in organs of mice injected with sub-lethal doses of cobra venom. The goal of this research is to observe the levels of ADA, collagen, histamine, IgE, myoglobin, and NGF in certain organs of mice injected with venom from the bee Apis mellifera.
Adult Balb/c female mice IM injected with half lethal dose of bee venom were sacrificed after 2 and 8 hours for removal of organs. The homogenates of the organs were assayed by ELISA for ADA, collagen, histamine, IgE, myoglobin, and NGF using respective antisera. Organs from mice injected with PBS were used as controls. It was observed that there were decreased levels of ADA, collagen, histamine, IgE, myoglobin, and NGF in certain organs after 2 h and tremendous decrease after 8 h. This is the first report showing the pharmacokinetics of ADA, collagen, histamine, IgE, myoglobin, and NGF as consequence of honeybee envenomation.
KEYWORDS: honeybee venom, adenosine deaminase, collagen, histamine, IgE, myoglobin, nerve growth factor.
INTRODUCTION
Snake venoms are known to cause different metabolic disorders, altering the cellular and enzymatic activities in animals and releasing pharmacological substances (24). There are some reports showing increments in cytokine levels in sera of mice and patients bitten by Bothrops asper and Bothrops jararaca (1) and in sera of mice injected with Bothrops atrox venom (2). Cytokine release in mouse model was reported in response to Bothrops asper envenomation (15). Petricevich et al. (16) reported increments in serum cytokine and nitric oxide production in mice injected with B. asper and B. jararaca venoms. Both venoms induced prominent elevations of tumor necrosis factors and interleukins IL-1, IL-6, IL-10 in sera of injected mice.
The extensive research by Rahmy et al. reported that liver and kidney of rabbits and cardiac muscles of rats were affected by Egyptian cobra venom injection (19, 20). Rahmy and Hemmaid (18) demonstrated the effects of intramuscular injection of a sub-lethal dose of the Egyptian cobra venom on the histological and histochemical pattern of rabbit kidney and liver. There are no published data regarding the effects of honeybee envenomation. This investigation reports that bee envenomation decreased the levels of pharmacological substances such as ADA, collagen, histamine, IgE, myoglobin, and NGF in certain organs of mice.
Adenosine deaminase (ADA) is an essential enzyme that controls the levels of biological active purines and 2'-desoxy adenosine in tissues and cells (6). ADA deficiency results in combined immunodeficiency by a severe T, B, and NK cell lymphopenia (7), and kidney, adrenal, and chondroosseous tissue alterations (22). ADA deficiency is also associated with bony and renal abnormalities, hepatocellular damage, neurological disorders, and pulmonary insufficiency (3). Some ADA-deficient patients have been reported to elevate IgE and eosinophilia, which are implicated in allergy-asthma (5).
Fagan et al. (4) reported that monoclonal antibodies to IgG4 induce histamine release from human basophiles in vitro. Evidence of histamine release was demonstrated as a principal pharmacological component of Australian wolf spider venom (21). The earliest demonstration of IgE antibodies against venoms in sera of victims of fatal anaphylaxis from stings has been reported (8). It was reported that both IgG and IgE antibody levels rose with immunotherapy with bee and wasp venoms (9). Zora et al. performed a study of the prevalence and clinical significance of venom-specific IgE (27).
As early as 1961, Reid (23) demonstrated that myoglobinuria is one of the characteristic symptoms in human snakebite victims. The presence of myoglobin in serum and urine was demonstrated as an early marker for kidney dysfunction (26). In mouse model, myoglobinuria due to envenomation by Pseudechis australis snake venom developed 60 minutes after venom injection, as indicated by red or dark urine (17)
Lipps (14) reported that the sub-lethal injection of cobra venom caused a decrease in levels of ADA, histamine, and IgE in organs of mice. Lipps (12) also reported decreased levels of NGF in organs of mice as consequence of sub-lethal injection of cobra venom.
MATERIALS AND METHODS
Honeybee venom
Honeybee (Apis mellifera) venom was purchased from Sigma-Aldrich CO (Catalogue no. V-3125).
Animals
Animals were used in compliance with the U. S. Public Health Service policy on humane care and use of animals. Rabbits and adult female Balb/c mice were purchased from Harlan Teklad, Madison, WI.
Production of polyclonal antibodies to IgE in rabbits
IgE was purchased from Scripps Laboratory. Anti-IgE was produced as follows: adult New Zealand rabbits received intramuscular injection (IM) of 250 µg of IgE per rabbit three times two weeks apart. The first injection consisted of a mixture of IgE and Freund's complete adjuvant (FCA). The subsequent injections consisted of the IgE mixed with Freund's incomplete adjuvant (FICA). Polyclonal antibodies against NGF were made in rabbits as described by Lipps (10). Anti-myoglobin made in rabbits was purchased from OEM Concepts, USA code# R3-C11.
Production of polyclonal antibodies to ADA, collagen, and histamine in mice
Antisera for ADA, collagen, and histamine are not commercially available. Antibodies against ADA and histamine were produced in mice as described by Lipps (13). ADA, collagen, and histamine were purchased from Sigma-Aldrich Co. Adult Balb/c mice were immunized by IM inoculation. First injection consisted of 0.2 ml/mouse containing 100 µg of ADA, collagen, or histamine in PBS mixed with equal volume of FCA. Subsequently, three additional injections were given two weeks apart, consisting of the same concentration mixed with FICA. Immunized mice were bled two weeks after the last injection through the ophthalmic vein and serum was collected.
Inoculation of mice with sub-lethal doses of bee venom
The lethal dose for bee venom was determined in mice by IM injection at various concentrations. The lethal dose for mouse was found to be 4 µg. Therefore, mice with similar weights were injected with 2 µg in 0.2 ml. Twenty mice were used, 5 injected with 0.2 mL PBS as controls.
Preparation of organ suspension
Bee venom-injected mice were divided into three groups of five and sacrificed for organs after 2 and 8 hours along with controls. The organs collected were brain, heart, kidney, liver, lung, muscle, pancreas, salivary gland, spleen, and ovary. Each type of organ from five mice was pooled. The pooled organs were homogenized in PBS. The homogenates were centrifuged and supernatants for each pool were separated. Protein concentration of the supernatants were adjusted to 100 µg/ml with PBS and frozen for further testing.
Enzyme-linked immunosorbent assay (ELISA)
ELISA tests were performed in 96-well microtiter plates (11). The reagents for ELISA were purchased from Sigma-Aldrich Co. The stocks from mice organs were diluted with carbonate-bicarbonate buffer, pH 9.4, to 10 µg/ml as coating antigen for ELISA. The wells of the plate were coated with antigen, 100 µl/well. The plate was left at room temperature (RT) overnight, after which it was emptied and washed three times (3x) with PBS, and the wells were blocked with 250 µl/well of 3% fish skin gelatin. After 30 min the plate was emptied and washed 3x with PBS. Antisera against ADA, collagen, histamine, IgE, myoglobin, and NGF diluted in gelatin were added. Three wells received 100 µl of each dilution of antiserum, diluted twofold from 1:300. The antigen controls without antibody were incorporated. The plate was incubated at 37°C in a humid incubator for 1 hour after which the plate was washed 3x with PBS. The horseradish peroxidase conjugated with mouse IgG was used for ADA, collagen, and histamine assay. For IgE, myoglobin, and NGF assay, horseradish peroxidase conjugated with rabbit IgG was used. Horseradish peroxidase conjugate at 100 µl/well was reacted for 30 min. Finally, after washing, the wells were reacted with O-phenylnediamine-HCl for color development. The plate was read after 30 min, and the OD was recorded at 405 mm as ELISA titer per 100 µl.
RESULTS
Bee venom-injected mice were sacrificed after 2 and 8 hours along with controls. The pooled homogenates of organs from five mice for each category were tested by ELISA for ADA, collagen, histamine, IgE, myoglobin, and NGF using respective anti-sera. The organ homogenates tested were brain, heart, kidney, liver, lung, muscle, pancreas, salivary gland, spleen, and ovary. The organs that showed considerable decreased levels for ADA, collagen, histamine IgE, myoglobin, and NGF are graphically illustrated in Figures 1 to 6.
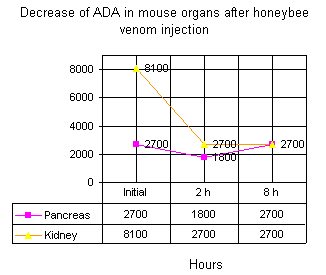
Figure 1 shows decreased levels of ADA in kidney and pancreas of mice injected with bee venom. The decreased ADA level in kidney was more prominent.
Figure 2 shows decreased levels of collagen in muscle and lung in mice injected with bee venom. Muscle is rich in collagen. Bee venom injection caused tremendous drop in collagen level in muscle, more at 8 h than at 2 h period.
Figure 3 shows decreased levels of histamine in heart and pancreas of mice injected with bee venom. The drop in histamine levels in heart and pancreas was considerable after 2 h post-injection of bee venom and remained the same after 8 h post-injection.
Figure 4 shows decreased levels of IgE in muscle and spleen of mice injected with bee venom. Spleen is known to be responsible for the production of immunoglobulins, including IgE. IM bee venom injection affected these organs causing tremendous drop in IgE levels.
Figure 5 shows decreased levels of myoglobin in brain and pancreas of mice injected with bee venom. In these experiments, IM injection of bee venom did not cause decreased levels of myoglobin for other organs. However, the drop of myoglobin in brain and pancreas was appreciable.
Figure 6 shows decreased levels of NGF in brain and liver as consequence of bee venom injection in mice. The NGF level in liver was sharply down at 2 h post-injection and remained steady until 8 h.
DISCUSSION
The aftermath of envenomation in experimental animals and human victims of snakebite is investigated by a variety of methods, using blood serum and only the kidneys and livers. Increments in the levels of various cytokines in serum, and pathological changes occurring in organs, liver and kidney as consequence of Egyptian cobra venom injection are reported (18,19,20). Increased glucose and lactate concentrations are known to be common during snake envenomation (24). Increased levels of various cytokines have been reported as consequence of snake and scorpion envenomation (25). However, the relationship between the increased levels of various cytokines and glucose in serum of experimental animals as consequence of envenomation and venom toxicity is not yet understood.
Recently, Lipps (12,14) reported the decreased levels of ADA, histamine, IgE, and NGF in organs of mice injected with a sub-lethal dose of Naja kaouthia venom. Assuming that what is lost from the organs must get into the circulation, it follows that ADA, histamine, IgE, and NGF should show increased levels in blood sera of mice like cytokines. However, assay of ADA, histamine, IgE, and NGF from sera of mice will require more complicated sandwich-type ELISA. Furthermore, one pooled serum sample will be assayed versus all different organs of mouse. Therefore, ELISA assays using organ homogenates are simple and straightforward. This assay system shows the drop in level in organs rather than increment in level in serum sample. It is a new reverse process.
This research showed the effect of sub-lethal injection of bee venom on various organs of mice in decreasing ADA, collagen, histamine, IgE, myoglobin, and NGF levels. The relationship between the decreased levels of ADA, collagen, histamine, IgE, myoglobin, and NGF in various organs of mice as consequence of sub-lethal injection of bee venom remains to be explored.
Received September 5, 2001
Accepted November 28, 2001
-
1BARRAVIERA B., LOMONTE B., TARAKOWSKI A., HANSON LA., MEIRA DA. Acute-phase reactions, including cytokines, in patients bitten by Bothrops and Crotalus snakes in Brazil. J. Venom. Anim. Toxins, 1995, 1, 11-22.
-
2BARROS SF., FRIEDLANSKAIA I., PETRICEVICH VL., KIPNIS TL. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Med. Inflm., 1998, 7,339-46.
-
3BOLLINGER ME., ARREDONDO-VEGA FX., SANTISTEBAN I., SCHWARZ K., HIRSHFIELD MS., LEDERMAN HM. Brief report: hepatic dysfunction as a complication of adenosine deaminase-deficiency. N. Engl. J. Med., 1996, 334, 1367-71.
-
4FAGAN DL., SLAUGHTER CA., CAPRA JD., SULIVAN TJ. Monoclonal antibodies to immunoglobulin G4 induce histamine release from human basophil in vitro. J. Allergy Clin. Immunol., 1982, 70, 399-404.
-
5FOZARD JR., PFANNKUCH HJ., SCHUURMAN HJ. Mast cell degranulation following adenosine A3 receptor activation in rats. Eur. J. Pharmacol., 1997, 298, 293-7.
-
6FREDERIKSEN S. Specificity of adenosine deaminase toward adenosine and 2'-deoxyadenosine analogues. Arch. Biochem. Biophys., 1966, 113, 383-8.
-
7HIRSCHHORN R. Immunodeficiency disease due to deficiency of adenosine deaminase. In: OCHS HD., SMITH CIE., PUCK JM. Eds. Primary immunodeficiency disease: a molecular and genetic approach. New YorK: Oxford University Press, 1999: 121-38.
-
8HOFFMAN DR., WOOD DL., HUDSON P. Demonstration of IgE and IgG antibodies against venoms in the blood of victims of fatal anaphylaxis. J. Allergy Clin. Immunol., 1983, 73, 193-6.
-
9KEMENY DM., LESSOF MH., PATEL S., YOULTEN LJ., WILLIAMS A., LAMBOURN E. IgG and IgE antibodies after immunotherapy with bee and wasp venom. Int. Arch. allergy Appl. Immunol., 1989, 88, 247-9.
-
10LIPPS BV. Biological and immunological properties of nerve growth factor from snake venoms. J. Nat. Toxins, 1998, 7, 121-30.
-
11LIPPS BV. Detection of nerve growth factor (NGF) in venoms from diverse source: Isolation and characterization of NGF from the venom of honeybee (Apis mellifera). J. Nat. Toxins, 2000, 9, 13-9.
-
12LIPPS BV. Decreased levels of nerve growth factor in organs of mice as a consequence of sub-lethal injection of cobra venom. J. Nat. Toxins, 10, 2001, 283-90.
-
13LIPPS BV., KHAN AA. The presence of pharmacological substances myoglobin and histamine in venoms. J. Venom. Anim. Toxins, 2001, 7, 45-55.
-
14LIPPS BV. Sub-lethal injection of cobra venom decreases adenosine deaminase, histamine, and IgE in organs of mice. J. Venom. Anim. Toxins, 8, 2002, 60-73.
-
15LOMONTE B., TARKOWSKI A., HANSON LA. Host response to Bothrops asper snake venom: analysis of edema formation, inflammatory cells and cytokine release in mouse model. Inflammation, 1993, 17, 93-105.
-
16PETRICEVICH VL., TEIXEIRA CFP., TAMBOURGI DV., GUTIERREZ JM. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon, 2000, 38, 1253-66.
-
17PONRAJ D., GOPALAKRISHNAKONE P. Establishment of an animal model for myoglobinuria by use of a myotoxin from Pseudchis australis (king cobra snake) venom in mice. Lab. Anim. Sci, 1996, 46, 393-8.
-
18RAHMY TR., HEMMAID KZ. Histological and histochemical alterations in the liver following intramuscular injection with sublethal dose of Egyptian cobra venom. J. Nat. Toxins, 2000, 9, 21-32.
-
19RAHMY TR., RAMADAN RA., FARID TM., EL-ASMAR MF. Renal lesions induced by cobra envenomation. J. Egypt. Ger. Soc. Zool., 1995, 17, 251-71.
-
20RAHMY TR., RAMADAN RA., FARID TM., EL-ASMAR MF. Hepatic pathogenesis due to cobra envenomation. J. Egypt. Ger. Soc. Zool., 2000, 16, 85-102.
-
21RASH LD., KING RG., HODSON WC. Evidence that histamine is the principal pharmacological component of venom from an Australian wolf spider (Lycosa godeffroyi). Toxicon, 1998, 36, 367-75.
-
22RATECH H., GRECO MA., GALLO DL., RIMOIN DL., KAMINO H., HIRCHHORN R. Pathologic findings in adenosine deaminase-deficiency severe combined immunodeficiency. Kidney, adrenal and chondro - osseous tissue alternations. Am. J. Pathol., 1985, 120, 157-69.
-
23REID HA. Myogloburia and sea-snake poisoning. Br. Med. J., 1961, 1, 1284-9.
-
24ROTHCHILD AM., ROTHCHILD Z. Liberation of pharmacologically active substances by snake venom. In: LEE CY Ed. Snake Venoms. Berlin: Springer-Verlag, 1979: 541.
-
25SOFER S., GUERON M., WHITE RM., LIFSHITZ M., APATE RN. Interleukin-6 release following scorpion sting in children. Toxicon, 1996, 34, 389-92.
-
26WU AH., LAIOS I., GREEN S., GORNET TG., WONG SS., PARMLEY L., TONNESEN AS., PLAISIER B., ORLANDO R. Immunoassays for serum and urine myoglobin: myoglobin clearance assayed as a risk factor for acute renal failure. Clin. Chem, 1994, 40, 796-802.
-
27ZORA JA., SWANSON MC., YUNGINGER JW. A study of the prevalence and clinical significance of venom-specific IgE. J. Allergy Clin. Immunol., 1988, 81, 77-82.
Publication Dates
-
Publication in this collection
16 Sept 2002 -
Date of issue
2002
History
-
Accepted
28 Nov 2001 -
Received
05 Sept 2001