Abstracts
The aim of this study was to adapt a mechanical procedure for the isolation of intact preantral follicles from Cebus apella ovaries. The interval effect of serial sections of the tissue chopper was tested on a number of preantral follicles isolated from ovaries (n=6) of three C. apella females, two prepubertal and one adult. Ovaries were divided into four equal parts and fragmented with a tissue chopper, adjusted for serial sections at intervals of 250, 500, 750 and 1,000µm, respectively. Isolated follicles were counted in a Neubauer's chamber and classified as primordial, primary or secondary. The number (mean±SE) of preantral follicles isolated from 1/4 ovary varied from 68,330+17,590 (at the 1,000µm cut interval) to 300,830+111,460 (at the 500µm cut interval. The mean diameter of the isolated preantral follicles varied from 11.6µm to 27.8µm.
monkey; preantral follicle; isolation; ovary; Cebus apella
O presente trabalho objetivou adaptar um procedimento mecânico para o isolamento de folículos pré-antrais a partir de ovários de Cebus apella. Para isso, foi testado o efeito do intervalo de cortes seriados do tissue chopper sobre o número de folículos pré-antrais isolados a partir de ovários (n=6) de três fêmeas de C. apella, duas pré-púberes e uma adulta. Os ovários foram divididos em quatro partes iguais e fragmentados com auxílio de um tissue chopper, ajustado para a realização de secções seriadas a intervalos de 250, 500, 750 e 1000µm, respectivamente. Os folículos isolados foram contados em câmara de Neubauer e classificados em primordiais, primários e secundários. O número (média ± EP) de folículos pré-antrais isolados de 1/4 de ovário variou de 68.330+17.590, no intervalo de corte de 1.000µm, a 300.830+111.460, no intervalo de corte de 500µm, o de melhores resultados. O diâmetro médio dos folículos pré-antrais isolados variou de 11,6µm a 27, 8µm.
macaco-prego; folículo; isolamento; ovário; Cebus apella
Mechanical isolation of capuchin monkey (Cebus apella) preantral ovarian follicles
Isolamento mecânico de folículos ovarianos pré-antrais de macaca-prego (Cebus apella)
S.F.S. DominguesI, II; H.S. FerreiraIII; J.A.P.C. MunizIII; A.K.F. LimaIV; O.M. OhashiV; J.R. FigueiredoIV; L.D.M. SilvaIV
IFaculdade de Medicina Veterinária, Universidade Federal do Pará, Belém, PA Rua Maximino Porpino da Silva, no 1000 68743-490, Castanhal, PA
IICentro de Ciências e Técnicas Agropecuárias, Universidade Estadual do Norte Fluminense, Campos dos Goytacazes, RJ
IIICentro Nacional de Primatas/FUNASA/MS, Ananindeua, PA
IVFaculdade de Medicina Veterinária, Universidade Estadual do Ceará, Fortaleza, CE
VCentro de Ciências Biológicas, Universidade Federal do Pará, Belém, PA
ABSTRACT
The aim of this study was to adapt a mechanical procedure for the isolation of intact preantral follicles from Cebus apella ovaries. The interval effect of serial sections of the tissue chopper was tested on a number of preantral follicles isolated from ovaries (n=6) of three C. apella females, two prepubertal and one adult. Ovaries were divided into four equal parts and fragmented with a tissue chopper, adjusted for serial sections at intervals of 250, 500, 750 and 1,000µm, respectively. Isolated follicles were counted in a Neubauer's chamber and classified as primordial, primary or secondary. The number (mean±SE) of preantral follicles isolated from 1/4 ovary varied from 68,330+17,590 (at the 1,000µm cut interval) to 300,830+111,460 (at the 500µm cut interval. The mean diameter of the isolated preantral follicles varied from 11.6µm to 27.8µm.
Keywords: monkey, preantral follicle, isolation, ovary, Cebus apella
RESUMO
O presente trabalho objetivou adaptar um procedimento mecânico para o isolamento de folículos pré-antrais a partir de ovários de Cebus apella. Para isso, foi testado o efeito do intervalo de cortes seriados do tissue chopper sobre o número de folículos pré-antrais isolados a partir de ovários (n=6) de três fêmeas de C. apella, duas pré-púberes e uma adulta. Os ovários foram divididos em quatro partes iguais e fragmentados com auxílio de um tissue chopper, ajustado para a realização de secções seriadas a intervalos de 250, 500, 750 e 1000µm, respectivamente. Os folículos isolados foram contados em câmara de Neubauer e classificados em primordiais, primários e secundários. O número (média ± EP) de folículos pré-antrais isolados de 1/4 de ovário variou de 68.330+17.590, no intervalo de corte de 1.000µm, a 300.830+111.460, no intervalo de corte de 500µm, o de melhores resultados. O diâmetro médio dos folículos pré-antrais isolados variou de 11,6µm a 27, 8µm.
Palavras-chave: macaco-prego, folículo, isolamento, ovário, Cebus apella
INTRODUCTION
Among the Brazilian endangered mammals, the following primate families are of particular note: Cebidae, Callimiconidae and Callithrichidae (Brasil, 1989). The cebids constitute a diverse group of animals subdivided into the following subfamilies: Cebinae, Pitheciinae and Aotinae. The Saimiri and Cebus, found within the subfamily Cebinae, are important neotropical nonhuman primates for biomedical research (Hearn, 1994). The Cebus apella (capuchin monkey) are the best neotropical primates at adapting to captivity, with annual reproduction of these animals being possible (De Luca et al., 1990). Thus, females of the capuchin monkey (C. apella) were chosen as experimental animals for the present study.
Considering the great importance of the neotropical primates as endangered species, it is necessary to develop captive breeding programs for these animals, not only for their preservation, but to obtain healthy individuals destined for biomedical research. Nowadays, in different parts of the world, the development of reproduction technologies has been a priority for many centers of research. Among these biotechnologies the manipulation of oocytes enclosed in preantral follicles, which seeks to isolate and culture preantral follicles, has the objective of optimizing the use of oocitary potential with high genetic value or endangered species, supplying a great number of oocytes for the in vitro maturation and fertilization techniques (Figueiredo et al., 1993).
The previous development of follicular isolation methods is necessary, in order the manipulation of oocytes enclosed in preantral follicles may be possible. At present, the developed methods can be divided into mechanical and/or enzymatic. The mechanical procedures have the advantage of offering a smaller damage risk to the follicular structure, being more economical, faster and more easily executed, when compared to the enzymatic methods. A great number of preantral follicles can be isolated using a tissue chopper adjusted for 50m m serial sections in bovine ovaries (Figueiredo et al., 1993). However, Lucci et al. (1999a) and Amorim et al. (2000b) verified that the section interval of the Tissue Chopper affects the number of isolated preantral follicles from goat and ovine ovaries.
At present there is no isolation method for ovarian preantral follicles from neotropical primates. Therefore, the objective of this study was to adapt a mechanical procedure of follicular isolation for the female capuchin monkey (C. apella), using as a model the mechanical procedure of follicular isolation described by (Figueiredo et al., 1993) for bovine ovaries. The influence of the serial section interval modification from tissue chopper on the number of isolated preantral follicles was verified to evaluate the isolation method efficiency.
MATERIALS AND METHODS
The females of C. apella were donated by the National Center of Primates (Belém-Pará) from the National Health Foundation (Brazil). Ovaries (n=6) from three females from two prepubertal and one adult C. apella females were used. All ovaries were obtained through ovariectomy. Chloridrate of ketamine (10mg/kg LW) and xylazine (1mg/kg LW) were used for chemical restrain. All females were kept under inhalator anesthesia (halothane 8%) during surgery and flumiscin-meglumine (25mg/kg LW) and enrofloxacine (10mg/kg LW) were administered for five days after surgical procedure. Immediately after the collection the ovaries were washed for 1-2 seconds in 70% alcohol and in 0.9% saline solution. During the journey to the laboratory, approximately 60 minutes, the ovaries were conserved in tubes containing buffered phosphate saline (PBS) and immersed in ice until the moment of follicular isolation. In the laboratory, structures, such as ligaments, were removed carefully from ovaries, and then, each ovary was divided into four fragments of similar weight. The ovarian fragments were maintained in PBS at 4°C until the moment of isolation.
The isolation of the preantral follicles was accomplished using the method described by Figueiredo et al. (1993) for bovine ovaries. This study tested the effect of changing the interval of serial sections in the tissue chopper on a number of preantral follicles isolated from C. apella ovaries. Each ¼ ovary was fragmented with a tissue chopper regulated to 250, 500, 750 or 1,000µm, respectively. After each treatment the isolated follicles were counted using a Neubauer's Chamber. The isolated follicles were classified using an optical microscope (×400) according to Hulshof et al. (1994). After the counting and follicular classification, 20 follicles of each category were measured with a micrometric eye lens on the inverted microscope (×400).
To evaluate the efficiency of the mechanical method used to recover the preantral follicles, two ovaries of C. apella, obtained by bilateral ovariectomy of an adult female, were divided approximately in two halves. One half was destined for the study of follicular population in situ, while the other half was used for the isolation with a tissue chopper, using the cut interval that produced the largest number of isolated preantral follicles. A half ovary submitted to histological analysis was fixed in Carnoy, sectioned serially at a thickness of 7µm and each 10th section of ovarian tissue fragment was mounted and stained with periodic acid Schiff followed by hematoxylin (PAS-hematoxylin). Each 10th section was evaluated under optical microscope (400×), with only follicles that presented a visible nucleus from the oocyte in the analyzed section being counted. The population of preantral follicles (PF) was estimated using the Fractionator Method (Gundersen et al., 1988).
The follicular dimensions were measured with a micrometric eye lens in an optical microscope (400×). The diameters of the follicles, oocyte and its nucleus from twenty primordial, primary and secondary follicles for ovary were measured. For the right and left ovaries, it was determinate the follicular recovery rate (FRR), using the following formula (Lucci et al., 1999b):
The averages of the isolated preantral follicles and determined through classic histology were represented as mean+SE, while the medium diameters of the follicles, oocytes and their nuclei were represented as mean+SD. The mean number (mean+SE) of isolated preantral follicles among the different treatments was compared using a Friedman's test. For comparison of the medium diameter of the isolated follicles among the different follicular categories, and the mean diameter of the follicles, oocytes and their nuclei of the preantral follicles present in situ, among the different follicular categories and between the right and left ovaries a Whitney-Mann's test was applied. Values were considered statistically significant when P<0.05.
RESULTS
All cut intervals produced a great number of isolated preantral follicles from ovaries of C. apella. However, there was a great variation in the number of isolated follicles in all treatments. The number (mean±SE) of preantral follicles isolated from 1/4 ovary varied from 68,330+17,590, at the 1,000µm cut interval, to 300,830+111,460, at the 500µm cut interval. The numbers (mean±SE) of isolated follicles at the 250 and 750µm intervals were 197,500+50,278 and 95,800+31,660, respectively. The mean number of isolated follicles at the 500µm interval was statistically superior to those all cut interval. The percentiles (mean+SE) of primordial, primary and secondary isolated follicles were 77.3%+2.4, 17.1%+2.1 and 5.6%+1.0, respectively.
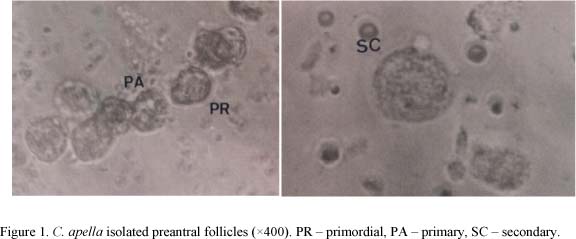
The isolated ovarian follicles presented almost spherical morphology, with one or more layers of granulosa cells surrounding the oocyte, without antrum and with clear coloration (Figure 1). The isolated follicles that presented oocytes and/or dark granulosa cells were considered as degenerated and, consequently, were not counted or measured. The medium diameter of the primordial, primary and secondary isolated follicles was 12.23+0.84µm, 15.10+0.95µm and 21.00+2.24µm, respectively. In isolated follicles, the follicular dimensions varied from 11.6µm to 27.8µm. The mean diameters of the primordial, primary and secondary follicles were significantly different from each other.
Table 1 shows the number of preantral follicles isolated in situ, and the follicular recovery rate (FRR) that was determined using the 500µm cut interval, since it isolated the largest mean number of preantral follicles. The mean follicular recovery rate was of 94.75±0.74%.
Concerning the morphology of the preantral follicles analyzed in histological cross-section, Figure 2 illustrates all follicular categories. The 15% and 9% percentages in the right and left ovaries, respectively, confirmed the occurrence of many follicles containing more than one oocyte (polyovular follicles).
The mean follicular diameters of the oocytes, their nuclei and of the preantral follicles in situ of the left and right ovaries are shown in the Table 2. A great variation in the parameters of both the right and left ovaries was observed. The mean diameters from follicles, oocytes, and their nuclei were statistically different from each other. However, there was no statistical difference between the right and left ovary in the mean diameters of primordial and secondary follicles. Thus mean diameters of follicles and oocytes from right ovarian primary follicles were statistically greater than in the left ovary, with no statistical difference between the diameters of the nuclei of oocytes from the primary follicles in the right and left ovaries being detected.
DISCUSSION
The results showed that it is possible to isolate a great number of preantral follicles from the Cebus apella female, using a simple mechanical procedure. Other mechanical methods for the isolation of preantral follicles have been described in the literature, by which some authors have isolated a great number of preantral follicles from the following species: cows (Figueiredo et al., 1993; Nuttinck et al., 1993; Hulshof et al., 1994), ewes (Amorim et al., 2000a,b), goats (Lucci et al., 1999a,b; Rodrigues et al., 1998) domestic cats (Jewgenow, Pitra, 1991; Jewgenow, Göritz, 1995) and wild cats (Jewgenow, Stolte, 1996; Jewgenow et al., 1997). Enzymatic procedures have been used for preantral follicle isolation in several species of mammals - hamsters (Roy, Greenwald, 1985), gilts (Greenwald, Moor, 1989), cows (Jewgenow, Pitra, 1991) and women (Roy, Treacy, 1993). Among these studies, only one had been developed for primates, and this was specifically an enzymatic method for isolation of human preantral follicles (Roy, Treacy, 1993). However, comparisons of these results with those of other authors are difficult to perform due to differences among species, methods (Greenwald, Moor, 1989; Hulshof et al., 1994, Jewgenow, Pitra, 1991; Nuttinck et al., 1993; Roy, Greenwald, 1985; Roy, Treacy, 1993) and follicular classification procedures (Roy, Greenwald, 1985; Roy, Treacy, 1993).
Figueiredo et al. (1993) developed a simple mechanical method for the isolation of bovine preantral follicle using a tissue chopper. However, these authors did not test the effect of different cut intervals of the tissue chopper on the number of follicles isolated from fetuses, calves and adult cows. These authors used the 50µm interval (personal communication). Rodrigues et al. (1998) used the same cut interval as Figueiredo et al. (1993) to isolate preantral follicles in caprine females. However, Lucci et al. (1999a) verified that there is an influence of the cut interval of the tissue chopper on the number of preantral follicles isolated from goat ovaries, and the interval that offers the largest number of isolated preantral follicles in the ovine species is 75µm. Amorim et al. (2000a) also demonstrated that there is influence of the cut interval of the tissue chopper on the number of preantral follicles isolated from ovine ovaries. In the same way as happens for the caprine and ovine species, it was possible in the present study to verify the influence of the tissue chopper cut interval on the number of preantral follicles isolated from C. apella ovaries.
A great variation in the number of preantral follicles isolated was observed in all treatments. These data are in accordance with other authors (Amorim et al., 2000a,b; Jewgenow, Göritz, 1995; Jewgenow, Stolte, 1996; Lucci et al., 1999a,b; Rodrigues et al., 1998). A variety of factors has been described which may affect the ovarian follicular population, including age (Peters, 1976), race (Cahill et al., 1979; Driancourt et al., 1985), reproductive stage (Erickson et al., 1976), nutrition (Scaramuzzi et al., 1993) and genetic factors (Cahill et al., 1979; Erickson, 1966). However, in the present study it was not aimed to verify the influence of these different factors on the number of isolated preantral follicles, but only to adapt a mechanical method for preantral follicular isolation in C. apella.
Concerning the percentage of isolated preantral follicles, the present results are similar to those mentioned in the literature, where most of the isolated preantral follicles are primordial. Carámbula (Carámbula, 1997) isolated 59%, 33.6% and 6.4% of primordial, primary and secondary follicles from bovine ovaries, respectively. Amorim et al. (2000a) isolated 92%, 6% and 2% of primordial, primary and secondary follicles from ovine ovaries, respectively, and Lucci et al. (1999a) isolated 96.3%, 2.5% and 1.2% of primordial, primary and secondary follicles in caprine ovaries, respectively. Thus it can be verified that in C. apella there is a higher percentage of primordial follicles than primary and secondary follicles.
There were statistical differences between the diameters of follicles among the different follicular categories, as much in isolated preantral follicles as in follicles present in situ. These data show that the follicular diameter may be used to differentiate primordial, primary and secondary follicles. These results are similar to those of Hulshof et al. (1994) in bovine ovaries. In relation to the diameters of follicles and oocytes from primary follicles, a statistical difference between the left and right ovary was observed. It can be suggested that the follicular development in the right ovary is faster than in the left ovary. Nagle et al. (1994) showed that the ovulation rates of the left and right C. apella ovaries are 62.5% and 37.5%, respectively. Such data are not in agreement with our results. However, comparison of the diameters of follicles, oocytes and their nuclei between right and left ovaries was performed in just one animal, and this characteristic may be specific to this C. apella female.
Although many studies show that preantral follicles can be isolated using mechanical and/or enzymatic methods, there is little information about their efficiency. Taking into consideration that the follicular population can be affected by the many factors previously mentioned in this discussion, it is necessary to use the same ovary or at least the contralateral ovary as control for the best evaluation of the isolation follicular method. The experimental design of the present work allowed evaluation of the efficiency of the isolation method of preantral follicles from C. apella, showing that it is possible to recover 85.3%±8.8 from the entire preantral follicular population from capuchins female using a tissue chopper regulated for the 500µm cut interval. Using this same experimental design, Amorim et al (2000b) obtained 5%, 28% and 26% FRR from fetuses, non-pregnant and pregnant ovine females, with a tissue chopper regulated for the 87.5µm cut interval. Lucci et al. (1999b) obtained 36.2% FRR, using a tissue chopper regulated for the 75µm cut interval for caprine ovaries. In the present study, the use of smaller cut intervals than 250µm destroyed the preantral follicles, while great intervals reduced the efficiency of mechanical dissociation of the ovarian fragments with pasteur pipettes, which in turn prevents preantral follicle release from the ovarian tissue.
During the in situ estimation of follicular population, the incidence of in situ polyovular follicles has been verified. In the literature, polyovular follicular occurrence has been reported in bitches (Mcdougall et al., 1997), gilts (Greenwald, Moor, 1989) and goats (Lucci et al., 1999b). Nuttinck et al. (1993) observed a case of a biovular follicle in the population of isolated bovine preantral follicles. During the growth of the embryonic ovary, the primordial germ cells (PGC) and the somatic cells are incorporated into the cortical ridge of the embryonic undifferentiated gonad (Erickson, 1966). After PGC invade the undifferentiated gonad, both they and the somatic cells undergo extensive hyperplasia. The germ cells lose their motile characteristic and proliferate rapidly. While they are undergoing mitosis, the somatic cells from the gonad are also proliferating rapidly (Hirshfield, 1991). The somatic cells intersperse themselves among the PGC, that initially were in clusters, gradually separating some of the others for the intervention of somatic cells (Rüsse, 1983). Lucci et al. (1999b) suggested that in goats, the incomplete separation of clusters from PGC could form the polyovular follicles. However, the formation of the polyovular follicles must be studied for the C. apella. Aurichio (1995) reported that C. apella females just produce one nestling for gestation, and this way the presence of polyovular follicles does not seem to affect the ovulation rate. In any case, the precise role of the polyovular follicles in capuchin and other mammalian females remains unknown.
In conclusion, the present work shows that a great number of ovarian preantral follicles may be isolated from capuchin monkey ovaries with success using a simple mechanical method, and the number of isolated follicles is affected by the tissue chopper cut interval.
The isolation, culture and conservation of preantral follicles may prove essential for the supply of viable oocytes for in vitro maturation and fertilization, to develop multiplication programs for endangered non-human primates in the future.
ACKNOWLEDGEMENTS
This study was supported by Centro Nacional de Primatas (CENP-FUNASA- Ananideua - PA) and the Laboratório de Fertilização in vitro (Universidade Federal do Pará, Belém-Brazil). Special thanks go out to all technician team of CENP (FUNASA).
Recebido para publicação em 4 de junho de 2002
E-mail: farhayldes@yahoo.com
- AMORIM C.A.; LUCCI C.M.; RODRIGUES A.P.R. et al. Quantitative and qualitative analysis of the effectiveness of a mechanical method for the isolation of preantral follicles from ovine ovaries. Theriogenology, vol.53, p.1251 - 1262, 2000.
- AMORIM, C.A.; RODRIGUES, A.P.R.; LUCCI, C.M. et al. Affect of sectioning on the number of isolated ovine preantral follicles. Small Rum. Res., vol.37, p.269-277, 2000.
- AURICHIO, P. Primatas do Brasil São Paulo: Terra Brasilis, 1995. 168p.
- BRASIL. Portaria nº 1.522, de 19 de dezembro de 1989. Lista Oficial de Espécies da Fauna Brasileira Ameaçada de Extinção, p.29- 55.
- CAHILL, L.P.; MARIANA, J.C.; MAULÉON, P. Total follicular populations in ewes of high and low ovulations rates. J. Reprod. Fertil., v.55, p.27-36, 1979.
- CARÁMBULA, S.F. Resgate de folículos pré-antrais de ovários de fetos bovinos e ovinos. 1997. Dissertação (Mestrado). Universidade Federal de Santa Maria, Santa Maria, RS.
- DE LUCA, R.R.; ALEXANDRE, S.R.; MARQUES, T. et al. Manual para técnicos em bioterismo. 2.ed. revisada e ampliada. São Paulo: H.A. Rothschild, 1990.
- DRIANCOURT, M.A.; CAHILL, L.P.; BINDON, B.M. Ovarian follicular populations and preovulatory enlargement in booroola and control merino ewes. J. Reprod., v.10, p.97-105. 1985.
- ERICKSON, B.H. Development and senescence of the postnatal bovine ovary. J. Anim. Sci., v.25, p.800-805, 1966.
- ERICKSON, B.H.; REYNOLDS, R.A.; MURPHREE, R.L. Ovarian characteristics and reproductive performance of the aged cow. Biol. Reprod., v.15, p.555-560, 1976.
- ERICKSON, G.F. An analysis of follicle development and ovum maturation. In: Seminars and reproductive endocrinology San Diego, California, p.233 - 254, 1986
- FIGUEIREDO, J.R.; HULSHOF, S.C.J.; VAN DEN HURK, R. Development of a combined new mechanical and enzimatic method for the isolation of intact follicles from fetal, calf and adult bovine ovaries. Theriogenology, v.40, p.789-799, 1993.
- GREENWALD, G.S.; MOOR, R.M. Isolation and preliminary characterization of pig primordial follicles. J. Reprod. Fertil., v.87, p.561-571, 1989.
- GUNDERSEN, H.; BAGGER, P.; BENDTSEN, T. The new sterological tools: dissector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS, v.96, p.857-881, 1988.
- HEARN, J. New world primates for research in human reproductive health. Am. J. Primatol., v.34, p.11-17, 1994.
- HIRSHFIELD, A.N. Development of follicles in the mammalian ovary. International review of cytology, 1991.
- HULSHOF, S.C.J.; FIGUEIREDO J.R.; BECKERS J.F. et al. Isolation and characterization of preantral follicles from fetal bovine ovaries. Vet. Q., v.2, p.78-80, 1994.
- JEWGENOW, K.; BLOTTNER, S.; LENGWINAT, T. et al. New methods for gamete rescue from gonads on nondomestic felids. J. Reprod. Fertil., Suppl., v.51, p.33-39, 1997.
- JEWGENOW, K.; GÖRITZ, F. The recovery of preantral follicles from ovaries of domestics cats and their characterization before and after culture. Reprod. Dom. Anim., v.39, p.183-193, 1995.
- JEWGENOW, K.; PITRA, C. Method for isolation from cattle ovaries. Reprod. Dom. Anim., v.26, p.281-289, 1991.
- JEWGENOW, K.; STOLTE, M. Isolation of preantral follicles from nondomestics cats - viability and ultrastructural investigations. Anim. Reprod. Sci., v.44, p.183- 193, 1996.
- LUCCI, C.M.; AMORIM, C.A.; BÁO, S.N. et al. Effect of the interval of serial sections of ovarian tissue in the tissue chopper on the number of isolated caprine preantral follicles. Anim. Reprod. Sci., v.56, p.39- 49, 1999a.
- LUCCI, C.M.; AMORIM, C.A; BÁO, S.N. et al. Study of preantral follicle population in situ and after mechanical isolation from undefined breed type goats at different reproductive stages. Anim. Reprod. Sci., v.56, p.223-236, 1999b.
- MCDOUGALL, K.; HAY, M.A.; GOODROWE, K.L. et al. Changes in the number of follicles an of oocytes in ovaries of prepurbetal, peripubertal and mature bitches. J. Reprod. Fertil., v.87, p.561-571, 1997.
- NAGLE, C.A.; DIGIANO, L.; PAUL, N. et al. Interovarian communication for the control of follicular growth and corpus luteun fuction in the cebus monkey. Am. J. Primatol., v.34, p.19-28, 1994.
- NUTTINCK, F.; MERMILLOD, P.; MASSIOP, A. et al. Characterization of in vitro growth of bovine preantral ovarian follicles: a preliminary study. Theriogenology, v.39, p.811-820, 1993.
- PETERS, H. The development and maturation of the ovary. Ann. Biol. Bioch. Biophys, v.16, p.271-278, 1976.
- RODRIGUES, A.P.R.; AMORIM, C.A.; LUCCI, C.M. et al. Isolamento Mecânico de folículos ovarianos pré-antrais em cabras. Ciênc. Rural, v.28, p.477-482, 1998.
- ROY, K.S.; TREACY, B.J. Isolation and long-term culture of human preantral follicles. Fertil. Steril., v.59, p.783-790, 1993.
- ROY, S.K.; GREENWALD, G.S. An enzymatic method for dissociation of intact follicles from the hamster ovary: Histological and quantitative aspects. Biol. Reprod., v.32, p.205-215, 1985.
- RÜSSE, I. Oogenesis in cattle and sheep. Bibl. Anat., v.24, p.77-92, 1983.
- SCARAMUZZI, R.J.; ADAMS, N.R.; BAIRD, D.T. et al. A model for follicle selection and the determimation of ovulation rate in the ewe. Reprod. Fertil. Dev, v.5, p.459-478, 1993.
Publication Dates
-
Publication in this collection
08 Oct 2003 -
Date of issue
June 2003
History
-
Received
04 June 2002