Abstract
The brain is a very expensive organ in metabolic terms. Each unit of brain tissue requires over 22 times the amount of metabolic energy as an equivalent unit of muscle tissue. There is no correlation across mammals, however, between the relative size of the brain and the relative basal metabolic rate. The Expensive Tissue Hypothesis explains this apparent paradox by looking at the metabolic cost of the brain in the context of the costs of other metabolically expensive organs in the body. The results show that the increase in brain size in humans is balanced by an equivalent reduction in the size of the gastro-intestinal tract. In other words, the increased energetic demands of a relatively large brain are balanced by the reduced energy demands of a relatively small gastro-intestinal tract. This relationship also seems to be true in non-human primates. The size of the gastro-intestinal tract is dependent on both body size and the quality of the diet. It is argued that humans (and other primates) could not have developed a relatively large brain without also adopting a high quality diet that would have permitted a reduction in the relative size of the gastro-intestinal tract. Dietary change is therefore viewed as a 'prime releaser' in brain evolution. It is argued that a high quality diet is necessary for the evolution of a relatively large brain. However, the change to such a high quality diet, which involved an increased proportion of animal based products, need not have been one of the 'prime movers' in brain evolution. In this context, and based on the archaeological and palaeoanthropological record, the factors most probably surrounding the evolution of the human brain are discussed.
REVIEW ARTICLE
Brains and guts in human evolution: The Expensive Tissue Hypothesis*
Leslie C. Aiello
*Conference presented at the 42o National Congress of Genetics, September 4-7, 1996, Caxambu, MG, Brasil.
Department of Anthropology, University College London, Gower Street, London WC1E 6BT England.
Tel: +44 171 380-856, Fax: +44 171 380-7728, E-mail: L.Aiello@ucl.ac.uk.
ABSTRACT
The brain is a very expensive organ in metabolic terms. Each unit of brain tissue requires over 22 times the amount of metabolic energy as an equivalent unit of muscle tissue. There is no correlation across mammals, however, between the relative size of the brain and the relative basal metabolic rate. The Expensive Tissue Hypothesis explains this apparent paradox by looking at the metabolic cost of the brain in the context of the costs of other metabolically expensive organs in the body. The results show that the increase in brain size in humans is balanced by an equivalent reduction in the size of the gastro-intestinal tract. In other words, the increased energetic demands of a relatively large brain are balanced by the reduced energy demands of a relatively small gastro-intestinal tract. This relationship also seems to be true in non-human primates.
The size of the gastro-intestinal tract is dependent on both body size and the quality of the diet. It is argued that humans (and other primates) could not have developed a relatively large brain without also adopting a high quality diet that would have permitted a reduction in the relative size of the gastro-intestinal tract. Dietary change is therefore viewed as a prime releaser in brain evolution. It is argued that a high quality diet is necessary for the evolution of a relatively large brain. However, the change to such a high quality diet, which involved an increased proportion of animal based products, need not have been one of the prime movers in brain evolution. In this context, and based on the archaeological and palaeoanthropological record, the factors most probably surrounding the evolution of the human brain are discussed.
Dietary quality has played a prominent role in theories of human evolution in general and the evolution of the human brain in particular. One of the most memorable of these theories is the Man the Hunter (Ardrey, 1961; Washburn and Lancaster, 1968). This theory argued that increasing amounts of meat in the hominid diet lead to increasing levels of cooperation among the males in the hunt, which lead to brain expansion and the associated development of cognition, language and symbolic culture. This hypothesis was fuelled by the realisation that an increase in the apparent consumption of meat correlated with the increase in brain size seen in Homo habilis and Homo erectus. It was also supported by the recognition in the archaeological record of the basic elements of a hunter-gatherer life-style (home bases and food sharing) (Isaac, 1971). Although the rather simplistic reasoning underlying the Man the Hunter hypothesis has lost favour in more recent years (eg. Tanner, 1981; Power and Aiello, in press) the importance of a high quality diet, and meat eating in particular, has been a common theme (eg. Foley and Lee, 1991; Leonard and Robertson, 1992, 1994).
Ideas of brain evolution centring on dietary quality have not been confined to humans and human evolution. Parker and Gibson, 1979 and Gibson, 1986 coined the Extractive Foraging Hypothesis to explain the relationship in primates. They argued that a relatively large brain correlates with omnivorous feeding in primates, which requires relatively complicated strategies for extracting high-quality foodstuffs. Alternatively, and in the context of primate frugivores, Milton (1979, 1981) and Clutton-Brock and Harvey (1980) suggested that relatively large brain size is associated with the need for a more sophisticated mental map for the location and exploitation of widely spread high quality food resources.
There is no doubt that there is an association between dietary quality and brain size across primates, including humans (Leonard and Robertson, 1994). However, how do we account for this association? There are two logical possibilities. The first is that there is a direct causal relationship between diet (and/or the associated ecological circumstances and foraging strategy) and brain size. Simplistically, hunting or extractive foraging or mental mapping would be directly selecting for large brain size. If this were true, then diet would be seen as a prime mover, or major selective agent, in the evolution of the brain (Falk, 1995). The alternative would be that diet is a prime releaser in brain evolution. In this sense, diet would release constraints on brain expansion and, given other selective agents or a combination of agents, permit brain expansion to take place.
The purpose of this contribution is to argue that diet must be viewed in the first instance as a prime releaser in brain evolution. Arguments will be presented to show that a relatively large brain requires a high quality, easy to digest diet. This conclusion does not necessary exclude diet as a prime mover in brain evolution, but it does not require it. Rather, it opens up the possibility of considering the importance of other selective agents (prime movers) in brain evolution without discounting the clear association between dietary quality and relative brain size (Leonard and Robertson, 1994). These other selective agents might include socio-ecological factors such as group size (Dunbar, 1992, 1993, 1994; Aiello and Dunbar, 1993) and social (or Machiavellian) intelligence (Byrne and Whiten, 1988) or a combination of these factors, as well as others which might include a clambering type of locomotion (Povinelli and Cant, 1995), or fully committed terrestrial bipedalism (Aiello, 1996a,b).
The expensive brain
The argument begins with the basic fact that the brain is a metabolically expensive organ. On the basis of in vivo determinations, the mass-specific metabolic rate of the brain is approximately 11.2 W/kg (watts per kilogram). This is over 22 times the mass-specific metabolic rate of skeletal muscle (0.4 W/kg) (Aschoff et al., 1971). A large brain would, therefore, be a considerable energetic investment. For example, an average human has a brain that is about 1 kg larger than would be expected for an average mammal of our body size (65 kg) and the metabolic cost of this brain would be just under 5 times that of the brain of the average mammal (humans = 14.6 watts, average mammal = 3.0 watts) (Aiello and Wheeler, 1995).
The energetic requirements of the growth and maintenance of a large brain have been frequently discussed in the literature (Martin, 1981, 1983; Armstrong 1982, 1983, 1985a,b, 1990; Hofman, 1983; Leonard and Robertson, 1992, 1994, 1996). The general interpretation has been that high quality food provides the extra energy to fuel the relatively large and expensive brain. Although this appears to be a clear and logical inference, there are serious problems with this argument. It would only work if large-brained animals ate the same quantity of high quality food as smaller-brained animals eat of lower quality food. This is clearly not the case for humans. Barton (1992) has shown that humans living a hunting and gathering lifestyle have a significantly lower daily food intake than smaller-brained non-human primates whose diet is of lower overall quality. Perhaps more importantly, there is no evidence that relatively large-brained animals have relatively high metabolic rates (MacNab and Eisenberg, 1989). In the particular case of humans, even though our brains are over a kilogram larger than would be expected for an average mammal of our body mass, we have the basal metabolic rate that would be expected for that average mammal (Aiello and Wheeler, 1995, Leonard and Robertson, 1994).
The Expensive Tissue Hypothesis
The main question is how we can have a relatively large brain without also having the relatively high basal metabolic rate that this relatively large brain would seem to require? The Expensive Tissue Hypothesis (ETH) provides an answer to this apparent contradiction (Aiello and Wheeler, 1995; Wheeler and Aiello, 1996). The ETH is based on the premise that the relationship between diet and energy requirements of the brain cannot be explained in isolation from the total energy requirements of the body. The important point is that the brain is not the only expensive organ in the body. There are four other expensive organs, the heart, the kidneys, the liver and the gastrointestinal tract. Together with the brain, these organs make up just under 7% of total body mass but they account for just under 70% of the total basal metabolic rate of the body (Table I). One way in which large-brained animals could maintain their relatively large brains without also having relatively high basal metabolic rates is to reduce the size (and thereby the metabolic cost) of some other expensive tissue in the body.
This possibility was tested by comparing the observed mass of each of the five expensive tissues in an average human (65 kg) with the expected mass for each of these organs in an average primate of human body mass (Aiello and Wheeler, 1995) (Figure 1). The observed mass of each of the human organs was taken from Synder (1975) and the expected organ masses for an average primate of human body mass were computed for the heart, liver, and kidneys on the basis of the least-squares regression equations for primates given in Stahl (1965). The expected brain mass and gut mass were determined on the basis of reduced major axis equations (Aiello and Wheeler, 1995). These equations are given in the caption of Figure 1.
Figure 1 - Observed and expected organ mass for a standard 65-kg human. Expected organ masses for the heart, liver and kidneys are from Stahl (1965). Heart mass: HW = 5.2*M0.987 (n = 321, r = 0.99); liver mass: LW = 32.2*M0.94 (n = 293, r = 0.98); kidney mass (both kidneys together): KW = 6.3*M0.87 (n = 268, r = 0.95). Expected brain size is based on the reduced major axis equation computed for higher primates (excluding humans) from data in Stephan et al. (1981). Brain mass: log10BW = 0.72 * log10M + 1.35 (N = 26, r = 0.98). Expected gut size is based on the reduced major axis equation computed for higher primates from data in Chivers and Haldik (1980, Chivers, personal communication; typesetting errors affecting data accuracy in Table 6 of Chivers and Haldik (1980) have been corrected and new species have also been added). Gut mass: Log10GM = 0.853 * Log10M - 1.271 (N = 22, r = 0.96). GM = gut mass in kilograms; W = other organ mass in grams; M = body mass in kilograms; n = number of individual animals; N = number of species; r = product moment correction coefficient. (After Aiello and Wheeler, 1995).
The combined mass of the metabolically expensive tissues for the reference adult human is very close to that expected for the average primate of human body size (observed human organ mass = 4.40 kg, expected organ mass = 4.45 kg), however the contributions of some of the individual organs to the total are very different. Human hearts and kidneys are just about as heavy as would be expected in an average primate of our body mass. However, the mass of the splanchnic organs (the liver and the gastro-intestinal tract) is approximately 900 g less than expected. Almost all of this shortfall is due to a reduction in the gastro-intestinal tract (781 g), the total mass of which is only about 60% of that expected for a similar-sized primate. The increase in mass of the encephalized human brain (850 g larger than would be expected) is more than compensated for by the reduction in the size of the splanchnic organs and particularly the gastro-intestinal tract. These are size relationships, but the mass specific organ metabolic rates of the brain and the splanchnic organs are virtually the same (Table I). This means that the basal metabolic cost of the relatively large human brain is almost perfectly balanced by the reduced metabolic cost of the smaller splanchnic organs, and particularly the gastro-intestinal tract.
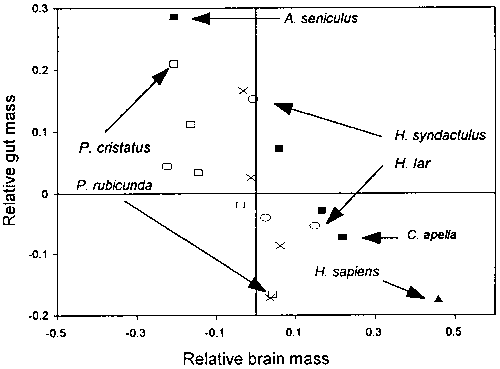
The balance between relative brain size and relative gut size also seems to be true for non-human primates (Aiello and Wheeler, 1995) (Figure 2). Those primates such as Alouatta seniculus, Presbytis cristatus, or Hylobates syndactulus that have relatively large guts also have relatively small brains while primates such as Cebus apella, Presbytis rubicunda, or Hylobates lar with relatively large brains also have relatively small guts.
Figure 2 - Relative brain size versus relative gut size in primates. Relative brain and gut sizes are determined on the basis of the higher primate equations given in Figure 1 and expressed as the residuals between the logged observed and expected sizes. The correlation of the residuals is -0.69 (n = 18, P < 0.001 for a one-tailed test). Filled squares = Cebids; open squares = colobines; open circles = hylobatids; X = other catarrhines. (After Aiello and Wheeler, 1995).
These relationships suggest that there has been a coevolution of brain size and gut size in humans and non-human primates. As the brain became larger in human evolution the gastro-intestinal tract became smaller. If this was the case, there is no reason that the basal metabolic rates (BMRs) of hominids or other encephalized primates would ever have been elevated above those typical of other primates as a consequence of the energetic costs of encephalization. On an intraspecific level and during the course of evolution, the expectation would be that encephalized individuals deviating from the ideal brain/gut-size relationships would also deviate in other aspects of their energy budgets. It would be highly likely that the elevated energetic requirements of these individuals would put them at a disadvantage in relation to conspecifics with the ideal brain/gut-size relationship and that this deviation would have had adverse consequence for their reproductive success. On an individual level this would be the selective mechanism driving the evolution of the observed brain/gut-size relationships. It is also clear that the inverse is true. If for ecological reasons animals have to have low quality, difficult to digest diets and correspondingly large guts, they cannot at the same time have relatively large brains.
When thinking of overall body size and energy requirements as well as the size and energy requirements of individual organs in the body, the relationship between relative brain size and relative gut size is perhaps not unexpected. Of all the expensive tissues only the brain and the gastro-intestinal tract have a significant latitude to vary in size in relation to overall body size of the animal. The size of the heart and kidneys are tied tightly to body size because of their function. The heart must be large enough to pump blood around the body and the kidneys large enough to produce urine of sufficient concentration. The size of the liver is also most probably tied closely to the size of the brain and therefore cannot vary inversely in relation to brain size. This is because the energy demands of the brain cannot exceed the capacity of the liver to store and ensure the uninterrupted supply of the glucose necessary to fuel brain metabolism.
It is also not possible to balance the energy requirements of the brain with the reduction in size of some tissue, such as skeletal muscle, which has a significantly lower mass-specific BMR. Because of the lower mass-specific BMR, a disproportionate amount of tissue would have to be replaced by an equal amount of tissue of no energetic cost whatsoever in order to balance the cost of the encephalized brain. In the specific case of skeletal muscle, 19 kg or 70% of the total muscle tissue would have to be replaced by inert tissue to balance the energetic requirements of the human brain (Aiello and Wheeler, 1995).
Diet and gut size
The gut is the only one of the expensive tissues that can vary in size sufficiently to offset the metabolic cost of the encephalized brain. This is because gut size is determined not only by overall body size but also by diet (Chivers and Haldik, 1980, 1984; Martin et al., 1985; MacLarnon et al,. 1986a,b; Martin, 1990). Gut size is associated with both the bulk and digestibility of food (Milton, 1986, 1993; Milton and Demment, 1988). Food of low digestibility requires relatively large guts with elaborated fermenting chambers (stomach and/or small intestine) while food of high digestibility (such as sugary fruits, protein and oil rich seeds and animal material) requires relatively smaller guts characterised by simple stomachs and proportionately long small intestines. For example, Presbytis rubicunda, which has a high-quality diet, contrasts sharply in relative gut size with P. cristatus, which has a much poorer-quality diet (Figure 2). The same is true of Hylobates lar, which spends more time feeding on fruits than on leaves and has a relatively smaller gut than H. syndactulus, which spends more time feeding on leaves than on fruits (Milton, 1987).
This strongly suggests that the observed association between diet quality and relative brain size (Parker and Gibson, 1979; Clutton-Brock and Harvey, 1980; Milton, 1987, 1988, 1993; Leonard and Robertson, 1992, 1994, 1996) is really a relationship between relative brain size and relative gut size, the latter being determined by dietary quality. The main conclusion is that no matter what is actually selecting for increase in brain size in humans and non-human primates, a high quality diet is necessary for encephalization. It relaxes the metabolic constraints on encephalization by permitting a relatively smaller gut, thereby reducing the considerable metabolic cost of this tissue.
The brain in human evolution
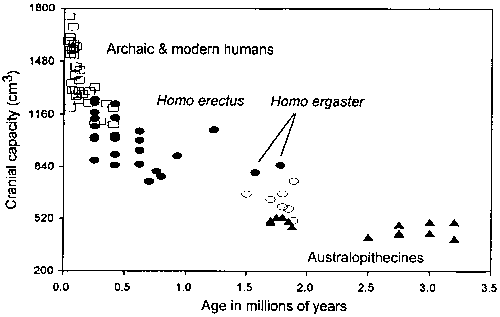
The change to a high quality diet, which involved increased reliance on animal products, appears to have been in place by the time of Homo ergaster (early Homo erectus). Homo ergaster has a cranial capacity of just over 800 cm3 (Aiello and Dean, 1990). This is about 70% larger than the average cranial capacity of adult australopithecines ( = 469 ± 46 cm3, n = 17; data from Aiello and Dean, 1990). This marked increase in cranial capacity occurred shortly after 2.0 mya (million years ago) and follows a long period of time during which australopithecine cranial capacity showed no significant increase (Figure 3). In relation to living primates, the australopithecines have relative brain sizes (in relation to inferred body mass) that fall either within or just above the upper range of the living primates (Aiello and Wheeler, 1995). This would suggest that the australopithecines had diets similar in quality to those of some of the most encephalized living primates. Indeed, Kay and Grine (1988) have pointed out the similarity in microwear patterns on the molar teeth of the robust australopithecines and those of Cebus, the most encephalized of all living primates that not only has a high-quality diet (Milton, 1987) but also resembles humans in its gut morphology (Martin et al., 1985; Milton, 1987). Strontium-calcium and stable carbon isotope ratios of Australopithecus robustus from Swartkrans (Member 1) support this interpretation by suggesting an omnivorous rather than strictly vegetarian diet for these hominids (Sillen, 1992; LeeThorp et al., 1994).
= 469 ± 46 cm3, n = 17; data from Aiello and Dean, 1990). This marked increase in cranial capacity occurred shortly after 2.0 mya (million years ago) and follows a long period of time during which australopithecine cranial capacity showed no significant increase (Figure 3). In relation to living primates, the australopithecines have relative brain sizes (in relation to inferred body mass) that fall either within or just above the upper range of the living primates (Aiello and Wheeler, 1995). This would suggest that the australopithecines had diets similar in quality to those of some of the most encephalized living primates. Indeed, Kay and Grine (1988) have pointed out the similarity in microwear patterns on the molar teeth of the robust australopithecines and those of Cebus, the most encephalized of all living primates that not only has a high-quality diet (Milton, 1987) but also resembles humans in its gut morphology (Martin et al., 1985; Milton, 1987). Strontium-calcium and stable carbon isotope ratios of Australopithecus robustus from Swartkrans (Member 1) support this interpretation by suggesting an omnivorous rather than strictly vegetarian diet for these hominids (Sillen, 1992; LeeThorp et al., 1994).
Figure 3 - The increase in absolute hominoid cranial capacity over time. Brain sizes from Aiello and Dean (1990), and ages from Aiello and Dunbar (1993). (After Aiello and Wheeler, 1995).
Homo ergaster, when compared to the earlier australopithecines, and particularly Australopithecus afarensis (AL 288-1) and Australopithecus africanus, not only has a much larger brain size but also very different body proportions (Aiello and Wheeler, 1995). In relation to these australopithecines, Homo ergaster as represented by the KNM-WT 15000 skeleton from Nariokotome, West Turkanna (Kenya) had a barrel-shaped thorax and a smaller pelvis, with a narrower abdominal region. By inference, the smaller abdominal region would have accommodated a considerably smaller gut relative to body size than the capacious abdominal region of the australopithecines. This would suggest a higher quality diet for these hominids. Direct evidence for this higher quality diet comes from a number of sources. Firstly, Homo ergaster body proportions suggest a more efficient adaptation to rapid locomotion, which has been interpreted by Shipman and Walker (1989) in the context of increased predatory behaviour in these hominids. Furthermore, recent archaeological analyses have suggested that hominid behavioural variability may have significantly increased beginning at about the time of the appearance of Homo ergaster (Rogers et al., 1994; Monahan, 1996). In particular, the archaeological evidence suggests that in comparison to earlier hominids, these later hominids were ...more capable predator/scavengers, regularly gaining early access to intact carcasses and diverse carcass resources, and more able to control specific locations on the landscape for extended durations. (Monahan, 1996, pp. 118-119). Sillen et al. (1995) have also reported relatively high strontium-calcium ratios for early Homo from Swartkrans which suggests that these hominids had a higher quality diet than the robust australopithecines, either incorporating more underground storage organs or the preferential consumption of animals having relatively high strontium-calcium ratios such as hyraxes.
The prime movers of hominid brain evolution
After the appearance of Homo ergaster brain size remains relatively constant until approximately 500,000 years ago (Figure 3).
This suggests that the factors selecting for brain size increase, the prime movers of hominid brain evolution in the Plio-Pleistocene, were specific to that period and have not operated continuously over the course of human evolution since 2 mya. Part of the brain size increase at this time was undoubtedly due to body size increase (McHenry, 1994). There is, however, good evidence that the brain also increased relative to body size (Kappelman, 1996). The question then becomes what features were unique to this period of hominid evolution that might be directly and causally related to the marked relative increase in brain size at this time?
The one notable feature in addition to the increase in the size of the brain that distinguishes Homo ergaster from the australopithecines is fully committed terrestrial bipedalism (Aiello, 1996a,b), and this is directly related to the increased aridity and seasonality characteristic of this part of the Plio-Pleistocene period in eastern Africa (Rogers et al., 1994). Terrestriality is associated with brain size increase because it implies both larger home-range sizes and larger group sizes for the hominids (Foley, 1987; Dunbar, 1992). Larger homeranges imply the need for more sophisticated mental maps of the territory and correspondingly larger brains to accommodate this, while larger group sizes put a premium on social, or Machiavellian intelligence (Byrne and Whiten, 1992; Byrne, 1996). Dunbar (1992, 1993; Aiello and Dunbar, 1993) has demonstrated that relative neocortex size, which is highly correlated with relative brain size in higher primates, is positively correlated with group size. There is also a positive correlation between amount of deceptive behaviour in primates and relative neocortical size (Byrne and Whiten 1992; Byrne, 1996).
This positive correlation between relative neocortex size and group size and deceptive behaviour does not mean that these are the only factors that may be related to the increase in hominid brain size, however (Barton, 1995; Aiello, 1996b). Bipedalism and the large size and elongated body form of Homo ergaster were very probably a necessary adaptation to thermoregulation in the hot African savannah (Wheeler 1991a,b, 1993; Aiello, 1996b). Committed bipedal locomotion would be related to brain expansion through the increased neural circuitry involved in enhanced speed and co-ordination of hand and arm movements. It would also be indirectly related to cognitive development and further brain expansion because the constricted bipedal pelvis would have necessitated the birth of less mature, altricial offspring, exposing them to a rich environment while the brain was still rapidly growing and developing.
It is highly probable that the combined effects of terrestriality (large group size and necessity for sophisticated mental mapping) and committed bipedalism (freeing of the forelimb and necessity for altricial births) were important prime movers in Plio-Pleistocene hominid brain evolution. Once these factors were in operation, the necessity for a high quality diet and correspondingly relatively small gut as prime releasers for brain expansion would become paramount. However, it is a mistake to see a high quality diet as solely a prime releaser for brain evolution (Aiello and Wheeler, 1995; Wheeler and Aiello, 1996). Barton (1995) has demonstrated that both group size and dietary type are independently correlated with relative neocortex size. This suggests that more complex foraging behaviour, particularly in a group context, may also be a prime mover in the evolution of the hominid brain and of cognitive abilities (Aiello and Wheeler, 1995).
In summary, then, although there is still much to learn about energy balance in humans and non-human primates, the Expensive Tissue Hypothesis allows us to account for the relationship between a high quality diet and relatively large brain sizes in humans and non-human primates in the first instance as a prime releaser for brain evolution. This provides the framework to then begin to understand the multifaceted factors that could have been behind the initial expansion of the hominid brain in the Plio-Pleistocene (Aiello, 1996a,b). These most probably include social intelligence, more sophisticated foraging behaviour necessitated by a high quality diet, and locomotor factors. They could also well include adaptation to climatic variability and the necessity for behavioural flexibility (Potts, 1996). The Expensive Tissue Hypothesis also illustrates the fact that the evolution of any organ of the body cannot profitably be studied in isolation. If we are going to understand the evolution of the brain we must first ask the question of how we can afford to have, and rely so completely on, such a large and expensive organ as the human brain? Only then can we begin to develop a balanced approach to the question of why the brain expanded in such a remarkable fashion during the course of human evolution.
ACKNOWLEDGMENTS
I would like to thank Professor Dr. Francisco A. Moura Duarte for inviting me to Brazil to present this paper at the 42o Congresso Nacional de Genética of the Sociedade Brasileira de Genética on the 5th of September, 1996, in Caxambu, Brazil. I would also like to thank Professor Peter Wheeler of the Liverpool John Moores University, England, who has collaborated with me over the past few years on the development of many of the ideas presented in this paper. I would also like to thank the following for discussion, criticism and collaboration on many of the ideas presented in this paper: Peter Andrews, Robert Barton, Robin Dunbar, Rob Foley, Kathleen Gibson, Catherine Key, Dan Nettle, Camilla Power, Todd Preuss, Alan Walker (who coined the name "The Expensive Tissue Hypothesis") and Bernard Wood.
REFERENCES
Aiello, L.C. (1996a). Hominine preadaptations for Language and Cognition. In: Modelling the Early Human Mind (Mellars, P. and Gibson, K., eds.). McDonald Institute Monographs, Cambridge, pp. 89-99.
Aiello, L.C. (1996b). Terrestriality, bipedalism and the origin of language. In: Evolution of Social Behaviour Patterns in Primates and Man (Maynard-Smith, J., ed.). Proc. Br. Acad. 88: 269-289.
Aiello, L.C. and Dean, M.C. (1990). An Introduction to Human Evolutionary Anatomy. Academic Press, London.
Aiello, L.C. and Dunbar R.I.M. (1993). Neocortex size, groups size and the evolution of language in the hominids. Curr. Anthropol. 34: 184-193.
Aiello, L.C. and Wheeler, P. (1995). The Expensive Tissue Hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36: 199-221.
Ardrey, R. (1961). African Genesis. Dell, New York.
Armstrong, E. (1982). A look at relative brain sizes in mammals. Neurosci. Lett. 34: 101-104.
Armstrong, E. (1983). Brain size and metabolism in mammals. Science 220: 1302-1304.
Armstrong, E. (1985a). Relative brain size in monkeys and prosimians. Am. J. Phys. Anthropol. 66: 263-273.
Armstrong, E. (1985b). Allometric considerations of the adult mammalian brain, with special emphasis on primates. In: Size and Scaling in Primate Biology (Jungers, W.L., ed.). Plenum, New York, pp. 115-146.
Armstrong, E. (1990). Brains, bodies and metabolism. Brain Behav. Evol. 36: 166-176.
Aschoff, J., Günther, B. and Kramer, K. (1971). Energiehaushalt und Temperaturregulation. Urban and Schwarzenberg, Munich.
Barton, R.A. (1992). Allometry of food intake in free-ranging anthropoid primates. Folia Primatol. 58: 56-59.
Barton, R.A. (1995). Neocortex size and behavioral ecology in primates. Proc. R. Soc. Lond. S. B Biol. Sci. 263: 173-177.
Byrne, R. (1996). Relating brain size to intelligence in primates. In: Modelling the Early Human Mind (Mellars, P. and Gibson, K., eds.). McDonald Institute Monographs, Cambridge, pp. 49-56.
Byrne, R. and Whiten, A. (Eds.) (1988). Machiavellian Intelligence. Clarendon, Oxford.
Byrne, R. and Whiten, A. (1992). Cognitive evolution in primates: evidence from tactical deception. Man 27: 609-627.
Chivers, D.J. and Haldik, C.M. (1980). Morphology of the gastrointestinal tract in primates. Comparisons with other mammals in relation to diet. J. Morphol. 166: 337-386.
Chivers, D.J. and Haldik, C.M. (1984). Diet and gut morphology in primates. In: Food Acquisition and Processing in Primates (Chivers, D.J., Wood, B.A. and Bilsborough, A., eds.). Plenum Press, New York, pp. 213-230.
Clutton-Brock, T.H. and Harvey, P.H. (1980). Primates, brains and ecology. J. Zool. 190: 309-323.
Dunbar, R.I.M. (1992). Neocortex size as a constraint on group size in primates. J. Hum. Evol. 22: 469-493.
Dunbar, R.I.M. (1993). Co-evolution of neocortex size, group size and language in humans. Behav. Brain Sci. 16: 681-735.
Dunbar, R.I.M. (1994). Neocortex size and group size in primates: a test of the hypothesis. J. Hum. Evol. 28: 287-296.
Falk, D. (1995). Comment on: The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36: 212-213.
Foley, R.A. (1987). Another Unique Species. Longman Scientific and Technical, London.
Foley, R.A. and Lee, P.C. (1991). Ecology and energetics of encephalization in hominid evolution. Philos. Trans. R. Soc. Lond. B 334: 223-232.
Gibson, K.R. (1986). Cognition, brain size and the extraction of embedded food resources. In: Primate Ontogeny, Cognition and Social Behaviour (Else, J.G. and Lee, P.C., eds.). Cambridge University Press, Cambridge, pp. 93-105.
Hofman, M.A. (1983). Energy metabolism, brain size and longevity in mammals. Q. Rev. Biol. 58: 495-512.
Isaac, G.L. (1971). The diet of early man: aspects of archaeological evidence from lower and middle Pleistocene sites in Africa. World Archaeol. 21: 278-299.
Kappelman, J. (1996). The evolution of body-mass and relative brain size in fossil hominids. J. Hum. Evol. 30: 243-276.
Kay, R.F. and Grine, F.E. (1988). Tooth morphology, wear and diet in Australopithecus and Paranthropus. In: Evolutionary History of the" Robust" Australopithecines (Grine, F.E., ed.). Aldine de Gruyter, New York, pp. 427-448.
Lee-Thorp, J.A., van der Merwe, N.J. and Brain, C.K. (1994). Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J. Hum. Evol. 27: 361-372.
Leonard, W.R. and Robertson, M.L. (1992). Nutritional requirements and human evolution: A bioenergetics model. Am. J. Hum. Biol. 4: 179-195.
Leonard, W.R. and Robertson, M.L. (1994). Evolutionary perspectives on human nutrition: The influence of brain and body size on diet and metabolism. Am. J. Hum. Biol. 6: 77-88.
Leonard, W.R. and Robertson, M.L. (1996). On diet, energy metabolism, and brain size in human evolution. Curr. Anthropol. 37: 125-128.
MacLarnon, A.M., Chivers, D.J. and Martin, R.D. (1986a). Gastro-intestinal allometry in primates including new species. In: Primate Ecology and Conservation (Else, J.G. and Lee, P.C., eds.). Cambridge University Press, Cambridge, pp. 75-85.
MacLarnon, A.M., Martin, R.D., Chivers, D.J. and Haldik, C.M. (1986b). Some aspects of gastro-intestinal allometry in primates and other mammals. In: Definition et Origines de lHomme (Sakka, M., ed.). Editions du C.N.R.S., Paris, pp. 293-302.
MacNab, B.K. and Eisenberg, J.E. (1989). Brain size and its relation to the rate of metabolism in mammals. Am. Nat. 133: 157-167.
Martin, R.D. (1981). Relative brain size and metabolic rate in terrestrial vertebrates. Nature 393: 57-60.
Martin, R.D. (1983). Human Brain Evolution in an Ecological Context (52nd James Arthur Lecture on the Evolution of the Human Brain). American Museum of Natural History, New York.
Martin, R.D. (1990). Primate Origins and Evolution. Chapman and Hall, London.
Martin, R.D., Chivers, D.J., MacLarnon, A.M. and Haldik, C.M. (1985). Gastrointestinal allometry in primates and other mammals. In: Size and Scaling in Primate Biology (Jungers, W.L., ed.). Plenum Press, New York, pp. 61-89.
McHenry, H. (1994). Behavioral ecological implications of early hominid body size. J. Hum. Evol. 27: 77-87.
Milton, K. (1979). Spatial and temporal patterns of plant foods in tropical forests as a stimulus to intellectual development in primates. Am. J. Phys. Anthropol. 50: 464-465 (Abstract).
Milton, K. (1981). Diversity of plant foods in tropical forests as a stimulus to mental development in primates. Am. Anthropol. 83: 534-548.
Milton, K. (1986). Digestive physiology in primates. News Physiol. Sci. 1: 76-79.
Milton, K. (1987). Primate diets and gut morphology: Implications for hominid evolution. In: Food and Evolution: Toward a Theory of Human Food Habits (Harris, M. and Boss, E.B., eds.). Temple University, Philadelphia, pp. 96-116.
Milton, K. (1988). Foraging behaviour and the evolution of primate intelligence. In: Machiavellian Intelligence (Byrne, R. and Whiten, A., eds.). Oxford Science Publications, Oxford, pp. 285-409.
Milton, K. (1993). Diet and primate evolution. Sci. Am. 269: 86-93.
Milton, K. and Demment, M.W. (1988). Digestion and passage kinetics of chimpanzees fed high and low fibre diets and comparison with human data. J. Nutr. 118: 1082-1088.
Monahan, C.M. (1996). New zooarchaeological date from Bed II, Olduvai Gorge, Tanzania: implications for hominid behavior in the Early Pleistocene. J. Hum. Evol. 31: 93-128.
Parker, S.T. and Gibson, K.R. (1979). A developmental model for the evolution of language and intelligence in early hominids. Behav. Brain Sci. 2: 367-407.
Potts, R. (1996). Evolution and climatic variability. Science 273: 922-923.
Povinelli, D.J. and Cant, J.G. (1995). Arboreal clambering and the evolution of self conception. Q. Rev. Biol. 70: 393-421.
Power, C. and Aiello, L.C. Female proto-symbolic strategies. In: Women in Human Origins (Hager, L.D., ed.). Routledge, London (in press).
Rogers, M.J., Harris, J.W.K. and Feibel, C.S. (1994). Changing patterns of land use by Plio-Pleistocene hominids in the Lake Turkana Basin. J. Hum. Evol. 27: 139-158.
Shipman, P. and Walker, A. (1989). The costs of becoming a predator. J. Hum. Evol. 18: 373-392.
Sillen, A. (1992). Strontium-calcium ratios (Sr/Ca) of Australopithecus robustus and associated fauna from Swartkrans. J. Hum. Evol. 23: 495-516.
Sillen, A., Hall, G. and Armstrong, R. (1995). Strontium calcium rations (Sr/Ca) and 87Sr/86Sr of Australopithecus robustus and Homo sp. from Swartkrans. J. Hum. Evol. 28: 277-285.
Stahl, W.R. (1965). Organ weights in primates and other mammals. Science 150: 1039-1042.
Stephan, H., Frahm, H. and Baron, G. (1981). New and revised data on volume of brain structures in insectivores and primates. Folia Primatol. 35: 1-29.
Synder, W.S. (1975). International Commission on Radiological Protection (ICRP). Report on the Task Force on Reference Man. ICRP pub. 23. Oxford University Press, Oxford.
Tanner, N.M. (1981). On Becoming Human. Cambridge University Press, Cambridge.
Washburn, S.L. and Lancaster, C.S. (1968). The evolution of hunting. In: Man the Hunter (Lee, R.B. and DeVore, I., eds.). Aldine, New York, pp. 293-303.
Wheeler, P.E. (1991a). The influence of bipedalism on the energy and water budgets of early hominids. J. Hum. Evol. 21: 117-136.
Wheeler, P.E. (1991b). The thermoregulatory advantages of hominid bipedalism in open equatorial environments: the contribution of increased convective heat-loss and cutaneous evaporative cooling. J. Hum. Evol. 21: 107-115.
Wheeler, P.E. (1993). The influence of stature and body form on hominid energy and water budgets: a comparison of Australopithecus and early Homo physiques. J. Hum. Evol. 24: 13-28.
Wheeler, P.E. and Aiello, L.C. (1996). On diet, energy metabolism, and brain size in human evolution - reply. Curr. Anthropol. 37: 128-129.
- Aiello, L.C. (1996b). Terrestriality, bipedalism and the origin of language. In: Evolution of Social Behaviour Patterns in Primates and Man (Maynard-Smith, J., ed.). Proc. Br. Acad. 88: 269-289.
- Aiello, L.C. and Dean, M.C. (1990). An Introduction to Human Evolutionary Anatomy Academic Press, London.
- Aiello, L.C. and Dunbar R.I.M. (1993). Neocortex size, groups size and the evolution of language in the hominids. Curr. Anthropol. 34: 184-193.
- Aiello, L.C. and Wheeler, P. (1995). The Expensive Tissue Hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36: 199-221.
- Ardrey, R. (1961). African Genesis Dell, New York.
- Armstrong, E. (1982). A look at relative brain sizes in mammals. Neurosci. Lett. 34: 101-104.
- Armstrong, E. (1983). Brain size and metabolism in mammals. Science 220: 1302-1304.
- Armstrong, E. (1985a). Relative brain size in monkeys and prosimians. Am. J. Phys. Anthropol 66: 263-273.
- Armstrong, E. (1990). Brains, bodies and metabolism. Brain Behav. Evol. 36: 166-176.
- Aschoff, J., Günther, B. and Kramer, K. (1971). Energiehaushalt und Temperaturregulation Urban and Schwarzenberg, Munich.
- Barton, R.A. (1992). Allometry of food intake in free-ranging anthropoid primates. Folia Primatol. 58: 56-59.
- Barton, R.A. (1995). Neocortex size and behavioral ecology in primates. Proc. R. Soc. Lond. S. B Biol. Sci. 263: 173-177.
- Byrne, R. and Whiten, A. (1992). Cognitive evolution in primates: evidence from tactical deception. Man 27: 609-627.
- Chivers, D.J. and Haldik, C.M. (1980). Morphology of the gastrointestinal tract in primates. Comparisons with other mammals in relation to diet. J. Morphol. 166: 337-386.
- Clutton-Brock, T.H. and Harvey, P.H. (1980). Primates, brains and ecology. J. Zool. 190: 309-323.
- Dunbar, R.I.M. (1992). Neocortex size as a constraint on group size in primates. J. Hum. Evol. 22: 469-493.
- Dunbar, R.I.M. (1993). Co-evolution of neocortex size, group size and language in humans. Behav. Brain Sci. 16: 681-735.
- Dunbar, R.I.M. (1994). Neocortex size and group size in primates: a test of the hypothesis. J. Hum. Evol. 28: 287-296.
- Falk, D. (1995). Comment on: The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36: 212-213.
- Foley, R.A. (1987). Another Unique Species Longman Scientific and Technical, London.
- Foley, R.A. and Lee, P.C. (1991). Ecology and energetics of encephalization in hominid evolution. Philos. Trans. R. Soc. Lond. B 334: 223-232.
- Hofman, M.A. (1983). Energy metabolism, brain size and longevity in mammals. Q. Rev. Biol. 58: 495-512.
- Isaac, G.L. (1971). The diet of early man: aspects of archaeological evidence from lower and middle Pleistocene sites in Africa. World Archaeol. 21: 278-299.
-
30Kappelman, J. (1996). The evolution of body-mass and relative brain size in fossil hominids. J. Hum. Evol. 30: 243-276.
- Lee-Thorp, J.A., van der Merwe, N.J. and Brain, C.K. (1994). Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J. Hum. Evol. 27: 361-372.
- Leonard, W.R. and Robertson, M.L. (1992). Nutritional requirements and human evolution: A bioenergetics model. Am. J. Hum. Biol. 4: 179-195.
- Leonard, W.R. and Robertson, M.L. (1994). Evolutionary perspectives on human nutrition: The influence of brain and body size on diet and metabolism. Am. J. Hum. Biol. 6: 77-88.
- Leonard, W.R. and Robertson, M.L. (1996). On diet, energy metabolism, and brain size in human evolution. Curr. Anthropol. 37: 125-128.
- MacNab, B.K. and Eisenberg, J.E. (1989). Brain size and its relation to the rate of metabolism in mammals. Am. Nat. 133: 157-167.
- Martin, R.D. (1981). Relative brain size and metabolic rate in terrestrial vertebrates. Nature 393: 57-60.
- Martin, R.D. (1983). Human Brain Evolution in an Ecological Context (52nd James Arthur Lecture on the Evolution of the Human Brain). American Museum of Natural History, New York.
- Martin, R.D. (1990). Primate Origins and Evolution Chapman and Hall, London.
- McHenry, H. (1994). Behavioral ecological implications of early hominid body size. J. Hum. Evol 27: 77-87.
- Milton, K. (1979). Spatial and temporal patterns of plant foods in tropical forests as a stimulus to intellectual development in primates. Am. J. Phys. Anthropol 50: 464-465 (Abstract).
- Milton, K. (1981). Diversity of plant foods in tropical forests as a stimulus to mental development in primates. Am. Anthropol 83: 534-548.
- Milton, K. (1986). Digestive physiology in primates. News Physiol. Sci. 1: 76-79.
- Milton, K. (1993). Diet and primate evolution. Sci. Am. 269: 86-93.
- Milton, K. and Demment, M.W. (1988). Digestion and passage kinetics of chimpanzees fed high and low fibre diets and comparison with human data. J. Nutr. 118: 1082-1088.
- Monahan, C.M. (1996). New zooarchaeological date from Bed II, Olduvai Gorge, Tanzania: implications for hominid behavior in the Early Pleistocene. J. Hum. Evol. 31: 93-128.
- Parker, S.T. and Gibson, K.R. (1979). A developmental model for the evolution of language and intelligence in early hominids. Behav. Brain Sci. 2: 367-407.
- Potts, R. (1996). Evolution and climatic variability. Science 273: 922-923.
- Povinelli, D.J. and Cant, J.G. (1995). Arboreal clambering and the evolution of self conception. Q. Rev. Biol. 70: 393-421.
- Rogers, M.J., Harris, J.W.K. and Feibel, C.S. (1994). Changing patterns of land use by Plio-Pleistocene hominids in the Lake Turkana Basin. J. Hum. Evol. 27: 139-158.
- Shipman, P. and Walker, A. (1989). The costs of becoming a predator. J. Hum. Evol. 18: 373-392.
- Sillen, A. (1992). Strontium-calcium ratios (Sr/Ca) of Australopithecus robustus and associated fauna from Swartkrans. J. Hum. Evol. 23: 495-516.
- Sillen, A., Hall, G. and Armstrong, R. (1995). Strontium calcium rations (Sr/Ca) and 87Sr/86Sr of Australopithecus robustus and Homo sp. from Swartkrans. J. Hum. Evol. 28: 277-285.
- Stahl, W.R. (1965). Organ weights in primates and other mammals. Science 150: 1039-1042.
- Stephan, H., Frahm, H. and Baron, G. (1981). New and revised data on volume of brain structures in insectivores and primates. Folia Primatol. 35: 1-29.
- Synder, W.S. (1975). International Commission on Radiological Protection (ICRP). Report on the Task Force on Reference Man ICRP pub. 23. Oxford University Press, Oxford.
- Tanner, N.M. (1981). On Becoming Human Cambridge University Press, Cambridge.
- Wheeler, P.E. (1991a). The influence of bipedalism on the energy and water budgets of early hominids. J. Hum. Evol. 21: 117-136.
- Wheeler, P.E. (1991b). The thermoregulatory advantages of hominid bipedalism in open equatorial environments: the contribution of increased convective heat-loss and cutaneous evaporative cooling. J. Hum. Evol. 21: 107-115.
- Wheeler, P.E. (1993). The influence of stature and body form on hominid energy and water budgets: a comparison of Australopithecus and early Homo physiques. J. Hum. Evol. 24: 13-28.
- Wheeler, P.E. and Aiello, L.C. (1996). On diet, energy metabolism, and brain size in human evolution - reply. Curr. Anthropol. 37: 128-129.
Publication Dates
-
Publication in this collection
13 Oct 1998 -
Date of issue
Mar 1997